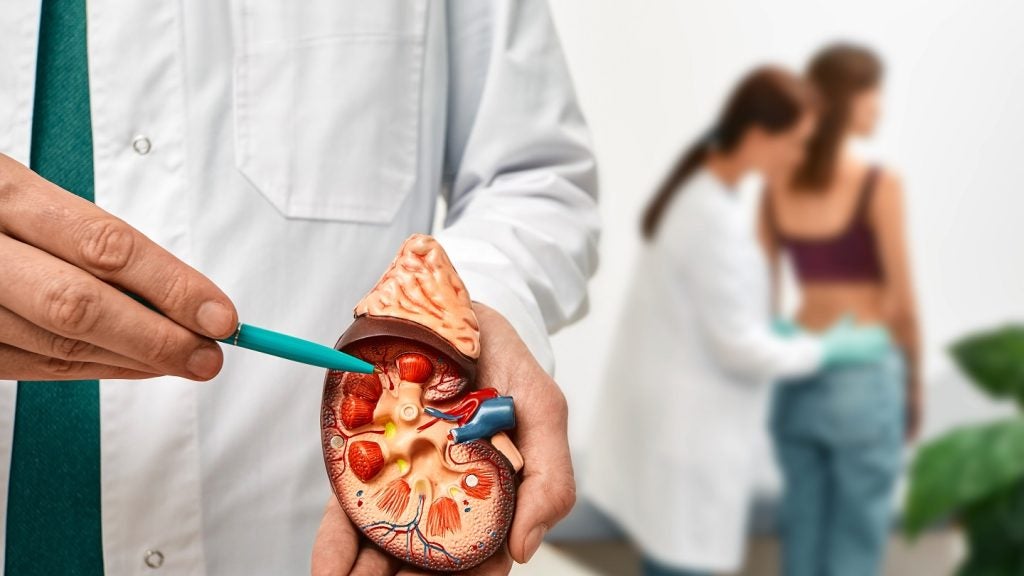
Chinook Therapeutics has reported results from a Phase I trial evaluating CHK-336, an oral small molecule LDHA inhibitor, in development for primary hyperoxaluria (PH) and other kidney stone disorders.
The placebo-controlled, single-centre, randomised, double-blinded study has evaluated the tolerability, safety, and pharmacokinetic (PK) profile of CHK-336 in 104 healthy volunteers (HV).
It includes single-ascending dose (SAD) and multiple-ascending dose (MAD) studies.
A single dose of CHK-336 ranging from 15mg to 500mg on day one was given to healthy volunteers against a placebo in the SAD portion of the study.
In the MAD portion, healthy volunteers daily received multiple doses of CHK-336 ranging from 30mg to 500mg or placebo for 14 days.
CHK-336 was found to be well tolerated in HVs who received single doses up to 500 mg and multiple doses up to 60mg for 14 days.
PK was also well characterised by dose-proportional exposures and a half-life supporting once-daily dosing of CHK-336.
One serious adverse event of anaphylaxis was observed in MAD cohort HV who received one 125mg dose of CHK-336.
Chinook Therapeutics chief scientific officer Andrew King said: “The data presented from the Phase I study of CHK-336 at this year’s ERA Congress successfully demonstrates hepatic LDH target engagement in healthy volunteers and establishes proof-of-mechanism for CHK-336 to decrease hepatic oxalate production.
“As we continue to investigate the SAE observed in the 125mg MAD cohort and consider the next steps, the CHK-336 programme will remain paused.”