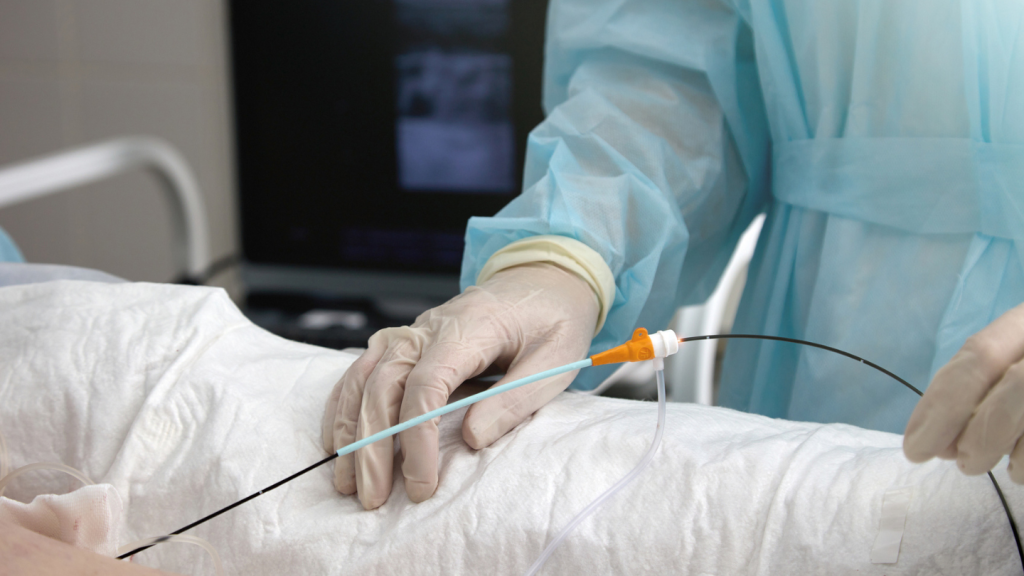
Citius Pharmaceuticals has reached an important milestone in the clinical trial for Mino-Lok therapy (MLT), an antibiotic lock solution to salvage catheters in patients with catheter-related bloodstream infections.
Citius believes all 92 events required to complete the trial have been achieved however, this is pending confirmation from independent reviewers. Some patients remain in active treatment.
MLT is a novel antibiotic lock therapy that combines minocycline with edetate disodium to treat patients with catheter-related bloodstream infections. Current treatment sees clinical removal and replacement of a central venous catheter (CVC), which the therapy provides an alternative to.
If approved, MLT, which Citius licensed from The University of Texas MD Anderson Cancer Centre, would be the first and only FDA-approved treatment that salvages central venous catheters causing central line-related bloodstream infections. MLT was granted qualified infectious disease product and fast track designations by the US' Food and Drug Administration (FDA). It has patent protection through 2024 and formulation patent protection through 2036.
Chairman and chief executive officer Leonard Mazur said: "This is a significant milestone for Citius as we approach completion of the Phase III Mino-Lok trial. As we complete therapy for patients in active treatment, we will continue to enrol patients in the pipeline and initiate shutdown activities.”
Mino-Lok Phase III trial design
The MLT Phase III trial (NCT02901717), which is being conducted in the US and India, is a multi-centre, randomised, open-label, blinded study to determine the efficacy and safety of MLT. The primary endpoint is the time to catheter failure. Additional secondary outcome measures include overall success, microbiological eradication, and clinical cure.
Patients who meet all necessary criteria for the study are randomised in a 1:1 ratio to receive either MLT or standard-of-care antibiotic lock therapy. Patients in the MLT arm receive a daily dose for seven days. For subjects in the control arm, the investigator determines the antibiotic used in the lock, dose, dwell time, and number of days of administration based on institutional standards or Infectious Diseases Society of America (IDSA) guidelines.