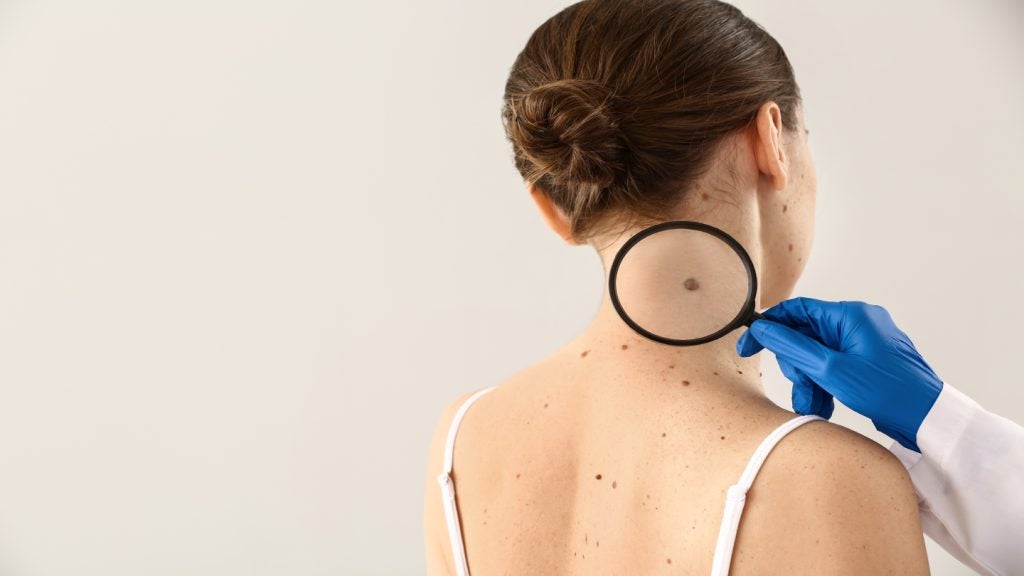
The California-based DermTech has announced positive results from its Trust 2 study, evaluating its system for ruling out melanoma showing a negative predictive value (NPV) of 99.7%.
DermTech reported positive topline results from the Trust 2 trial, which examined the gene expression assay component of the DermTech Melanoma Test (DMT).
The study results demonstrated an NPV of 99.7% for the foundational gene expression assay component of the company’s melanoma test. The study results also included a sensitivity of 95.8%, a specificity of 69.4% and a positive predictive value (PPV) of 13.4%.
Launched in 2021, the Trust 2 study is a follow-up to the company’s previous real-world study Trust 1. The first trial was published in 2021, examining the evaluated tested lesions of more than 1,500 patients.
The Trust 2 study enrolled more than 20,000 patients previously tested with DMT in a real-world clinical setting. The follow-up evaluations occurred for 5,000 tested lesions with median and mean follow-up durations of 348 days and 337 days, respectively.
These follow-up examinations included pathology diagnoses for lesions that were previously biopsied. Also, it included re-examination for lesions that were monitored rather than biopsied, in which the lesion was classified as either stable or changed in a manner determined as concerning for melanoma.
Loren Clarke, chief medical officer for DermTech, said: “A high NPV delivers assurance that a suspicious pigmented lesion which tests negative is unlikely to be a melanoma. As a non-invasive test that has demonstrated an NPV of 99% or higher in multiple, large studies, the DMT provides actionable genomic information for a suspicious pigmented lesion that a clinician may be hesitant to biopsy for a variety of reasons.”
GlobalData’s Medical Device Intelligence Centre details how the market for cancer screening tests is set to hit $1.8bn by the end of this year, with that figure expected to rise to $2.17bn by the end of 2030.
GlobalData is the parent company of Clinical Trials Arena.
In November 2023, competitor DermaSensor announced results from a study examining its own artificial intelligence-powered system for detecting skin cancers.