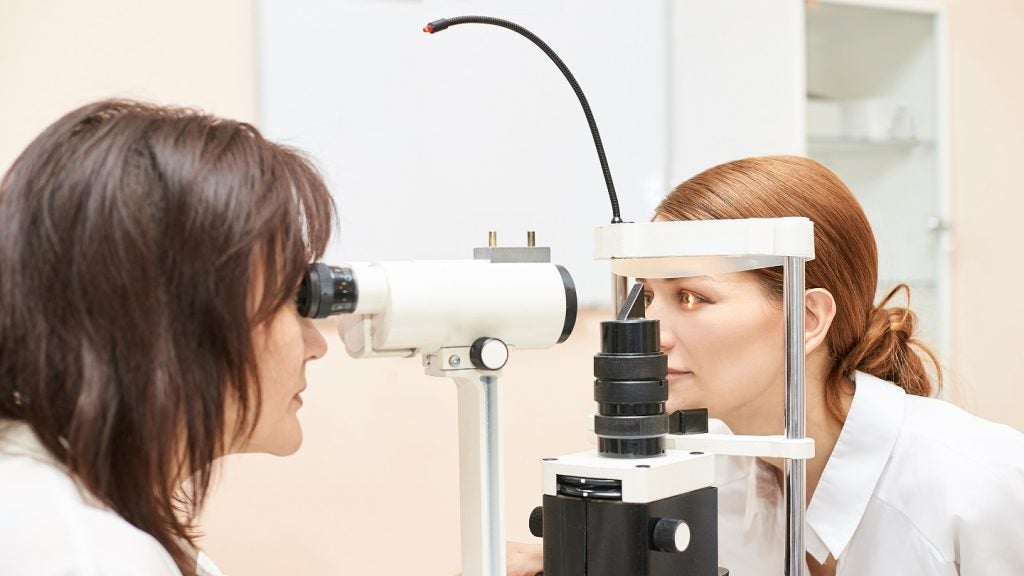
Eyebiotech (EyeBio) has dosed the first participants with diabetic macular oedema (DME) and neovascular age-related macular degeneration (NVAMD) in a Phase Ib/II clinical trial of intravitreal (IVT) Restoret (EYE103), a tri-specific Wnt agonist antibody.
The two-part, multi-centre study is titled ‘Anti-permeability Mechanism and Age-Related Ocular Neovascularization Evaluation (AMARONE).’
It includes an open-label multiple-ascending dose (MAD) study to assess the safety of Restoret and a single-masked, dose-finding study to compare the safety and preliminary efficacy of the therapy in NVAMD and DME participants.
Restoret will be administered to up to 92 subjects as part of a 12-week regimen.
Safety and mean change in best-corrected visual acuity (BCVA) from baseline to week 12 are the primary endpoints.
EyeBio chief scientific officer Tony Adamis said: “Restoret has the potential to address a major unmet need in these diseases, namely excess fluid in the retina.
“Anti-VEGF registrational trials and real-world data both show that excess fluid leads to poorer vision, and the longer that fluid resides in the retina, the less opportunity there is to recover vision.
“Restoret addresses a new biology, which is critical if we want to deliver results beyond those seen with anti-VEGF alone.”
The first participants dosed in the study were treated by Mark Barakat of Retinal Consultants of Arizona and J Edward Ysasaga of Southwest Retina Specialists.
EyeBio chief medical officer Jonathan Prenner said: “More than 60% of patients have suboptimal outcomes and more than 30%have active disease despite treatment with anti-VEGF agents.
“Restoret may present an opportunity to break through the efficacy ceiling we have reached with an anti-VEGF standard of care.”