A drug-coated balloon that helps restore blood vessels via light activation has been approved for use in US clinical trials, following an investigational device exemption (IDE) by the US Food and Drug Administration (FDA).
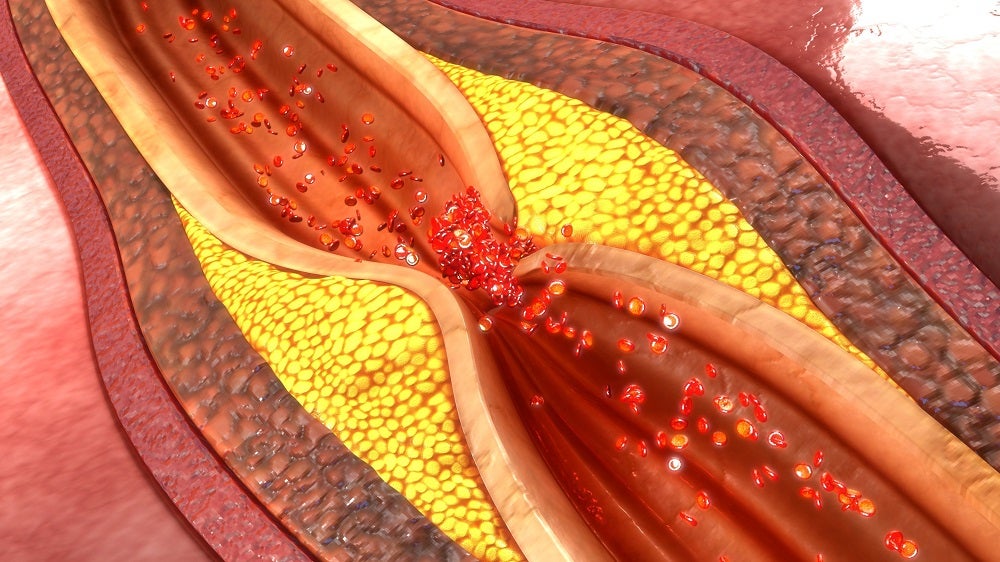
Alucent Biomedical’s light-activated, drug-coated balloon catheter technology creates a natural, stent-like scaffold that harnesses structures and processes already present in the vessel wall to treat peripheral artery disease and help haemodialysis patients with arteriovenous fistula maturation.
The intravascular system, called Alucent Natural Vessel Scaffolding (NVS), combines traditional angioplasty techniques with a photoactivated small molecule compound. The drug diffuses into the vessel wall from the balloon and then is photoactivated by a light fibre form within the balloon. The small molecule compound helps create extracellular matrix protein linking in the vessel walls.
In a statement announcing the IDE, Alucent Biomedical said that the technology avoids potential complications from permanent implant approaches, whilst still promoting long-lasting vascular remodelling to help with sustained blood flow improvement.
The device is currently being tested in feasibility studies in Poland and Australia. In April 2022, the Utah, US-based company started enrolment for a trial investigating the device in patients with peripheral artery disease. In January 2023, Alucent Biomedical enrolled the first patient in a trial evaluating the device for arteriovenous fistula (AVF) in people with end stage renal disease (ESRD) in Australia.
The company expects enrolment for those two trials to conclude by the end of 2023, and it is eyeing the first trial for the device in the US. Approximately 6.5 million people ages 40 and above in the US have peripheral artery disease.
Dr. Myles Greenberg, Alucent Biomedical’s CEO said: “IDE approval by the FDA’s Division of Coronary and Peripheral Interventional Devices is another validation of our novel approach to treating vascular disease. AlucentNVS technology is poised to change the standard of care in treating patients undergoing life- and limb-saving vascular procedures.”