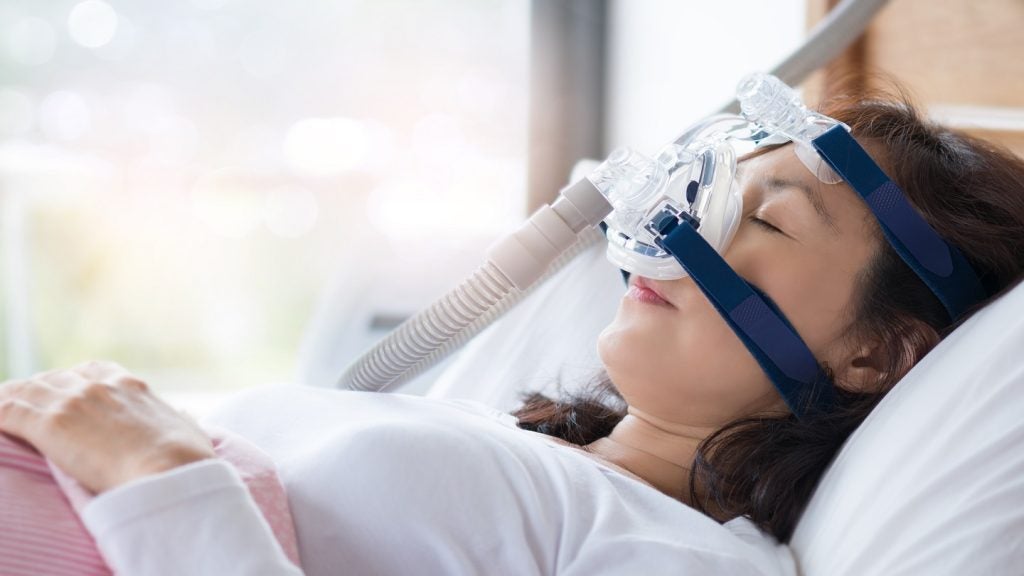
The US Food and Drug Administration (FDA) has granted clearance to Incannex Healthcare’s investigational new drug (IND) application enabling the initiation of a Phase II/III study of IHL-42X for the treatment of obstructive sleep apnoea.
The FDA granted the approval after completing its review of the substantial application submitted to the authority on 20 July.
Incannex’s pivotal trial will evaluate the effect of IHL-42X in obstructive sleep apnoea patients who are intolerant, non-compliant, or naive to positive airway pressure treatment, administered via CPAP devices.
Patients will receive one dose of IHL-42X, dronabinol, acetazolamide or placebo.
They will complete daily surveys on their sleep quality and attend the clinic monthly for their sleep and cognitive function assessments and other safety and efficacy measures.
A polysomnography will also be conducted overnight in patients every three months to determine the effect of treatment on the Apnoea-Hypopnea Index (AHI) along with various other sleep parameters.
IHL-42X is a combination of dronabinol, a synthetic form of tetrahydrocannabinol (THC), and acetazolamide, a carbonic anhydrase inhibitor.
In earlier Phase II proof of concept study, IHL-42X was found to reduce AHI by an average of 50.7% versus baseline assessments.
The concentrations of THC in blood were also observed to be below the limits for impaired driving the morning after nocturnal IHL-42X dose administration.
Furthermore, no serious treatment-emergent adverse events were reported during the study.