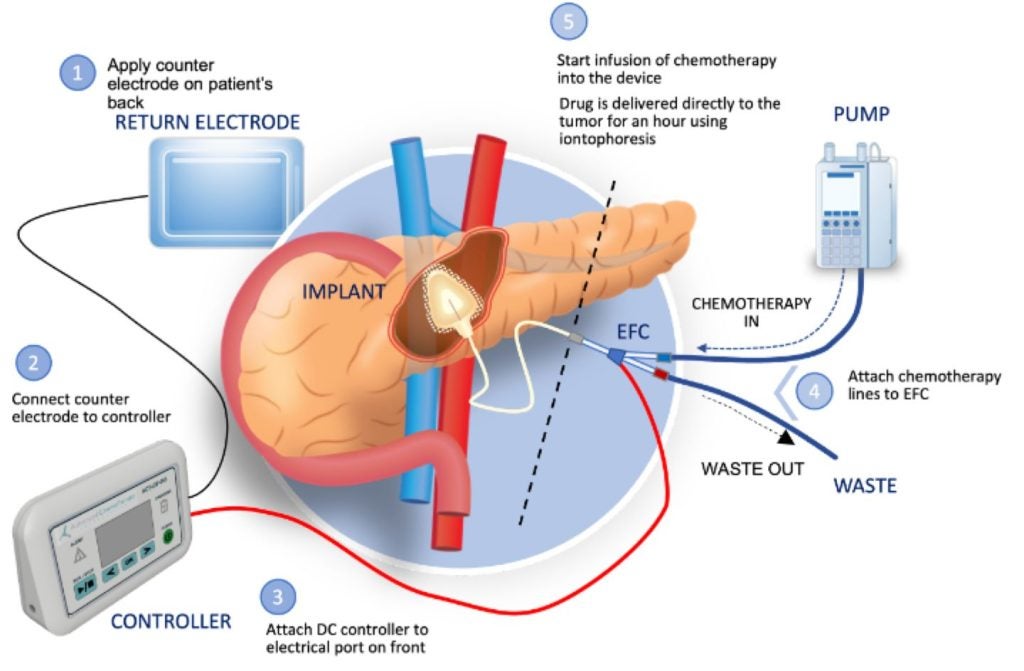
Biopharmaceutical company Focal Medical has received clearance for its investigational new drug (IND) application from the US Food and Drug Administration (FDA) to commence a Phase Ib clinical trial of its targeted therapeutic product, ACT-IOP-003, for pancreatic cancer.
This implantable iontophoretic product can deliver high levels of gemcitabine directly to the pancreas, potentially reducing systemic drug exposure and toxicity.
ACT-IOP-003 has shown promising results in orthotopic, patient-derived xenograft murine models of human pancreatic cancer, achieving a significant 40% reduction in tumour volume.
The modified dose escalation, open-label, multicentre trial aims to evaluate the tolerability, safety, and clinical activity of the product in patients with locally advanced nonresectable (LANR) pancreatic cancer.
Anticipated to begin in mid-2024, the trial will involve up to 12 subjects across two cohorts.
Participants will receive treatment once or twice a week over eight weeks.
Focal Medical is focused on the development of products based on its patented iontophoresis delivery system.
The company's technology seeks to overcome the challenges of traditional systemic drug delivery, such as systemic toxicity and barriers to therapeutic effect.
Focal Medical is also expanding its product pipeline to include therapies for other solid tumours and genomic medicine products.
Focal Medical chief technology officer Dr William Daunch said: “Once a locally advanced tumour is not resectable, treatment options are limited and these patients experience a significantly reduced survival outlook compared to resectable cases.
“Our thesis, which we have demonstrated in animal models of pancreatic cancer, is that localised delivery of high concentrations of gemcitabine via our implantable iontophoretic device can reduce tumour volume to a point where surgical removal may be possible while also minimising systemic exposure and associated toxicity.
“If successful, we may offer the opportunity of extended survival for the significant number of pancreatic cancer patients presenting with nonresectable disease.”