Gilead Sciences has announced the US Food and Drug Administration’s decision to pause screening and enrolment of patients in US studies investigating the treatment of acute myeloid leukaemia (AML) with magrolimab.
The partial clinical hold follows the discontinuation of a Phase III trial investigating the same drug combined with azacitidine in patients with higher-risk myelodysplastic syndromes (MDS). Gilead shelved the study following poor efficacy results, not due to safety concerns.
Despite the FDA's hold on the AML studies, which include evaluating therapies in combination with venetoclax and azacitidin, patients who have already been enrolled can continue to receive treatment. The FDA action only pertains to AML studies, with Gilead stating that magrolimab studies in solid tumours can continue as planned.
In a statement announcing the clinical hold, Gilead said it “is working with regulatory authorities to determine next steps to release the partial clinical hold for new patient enrolment in the magrolimab AML studies”.
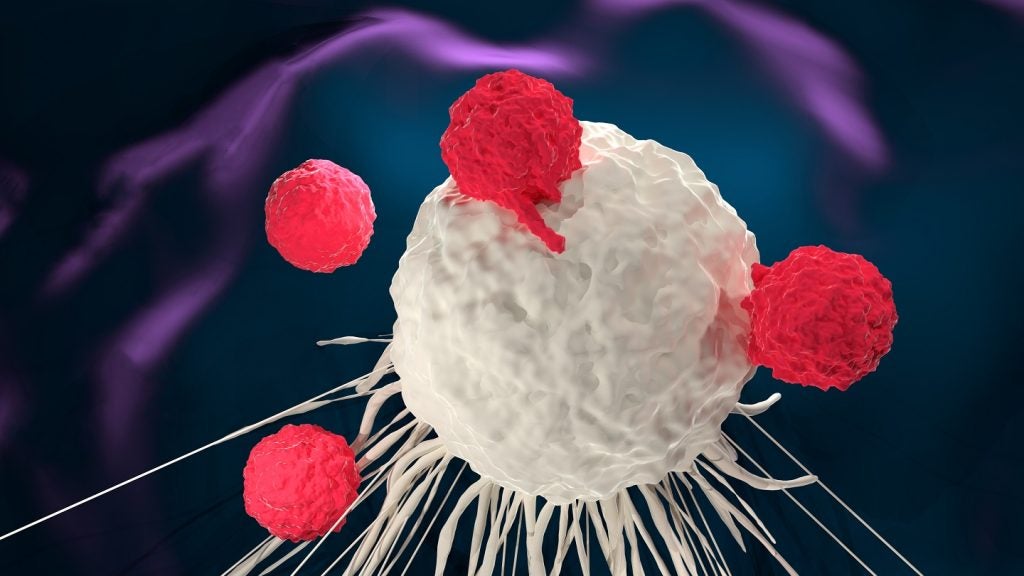
Gilead gained the candidate when it acquired Forty Seven for $4.9bn in 2020. Magrolimab is an investigational immunotherapy that works by blocking the inhibitory CD47-signal regulatory protein (SIRPα) interaction.
This enhances the ability of the body’s immune cells to fight malignant cells. By removing the signalling safety net of cancer cells, macrophages and phagocytes are able to identify and destroy them. The FDA hold delays the candidate’s route to be a potential first-in-class investigational anti-CD47 immunotherapy.