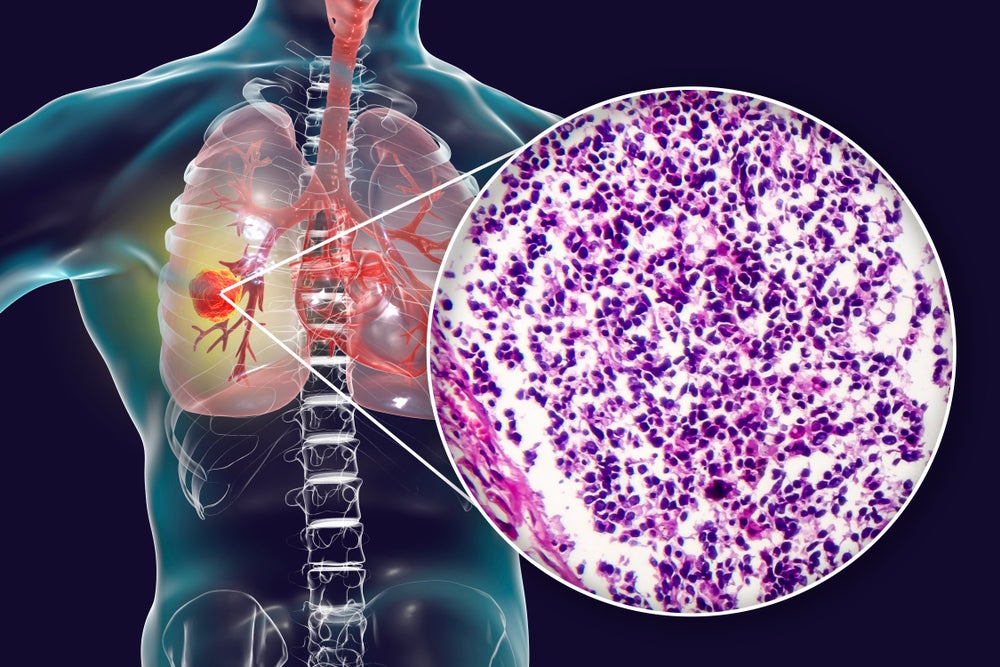
The US Food and Drug Administration (FDA) has granted clearance to TME Pharma’s investigational new drug (IND) application enabling the initiation of the Phase II OPTIMUS study of NOX-A12 to treat pancreatic cancer.
The open-label study will assess the efficacy and safety of NOX-A12 along with Merck’s Keytruda (pembrolizumab), and two different chemotherapy regimens including nanoliposomal irinotecan/5-FU/Leucovorin or gemcitabine/nab-paclitaxel.
This combined therapy is intended to be used as second-line treatment in microsatellite-stable metastatic pancreatic cancer patients.
The study intends to enrol nearly 70 patients from clinical sites in France, Spain and the US. The trial has previously received approval in Spain and France.
TME Pharma CEO Aram Mangasarian said: “Now we will be able to test NOX-A12 in clinical trials in the US, and this is a very positive piece of news for the future development of NOX-A12.
“We have thus delivered on our promise to the market to bring the OPTIMUS IND application with the FDA to successful completion, allowing rapid resumption of the pancreatic cancer programme once financing is available.”
OPTIMUS is carried out as part of a second collaboration with Merck which will supply pembrolizumab, an anti-PD-1 therapy, for the trial.
TME Pharma is also evaluating NOX-A12 combined with radiotherapy and with or without bevacizumab in the GLORIA Phase I/II trial of glioblastoma, a brain cancer.
Interim data demonstrated encouraging efficacy signs showing an 83% rate of survival at 14 months in patients.