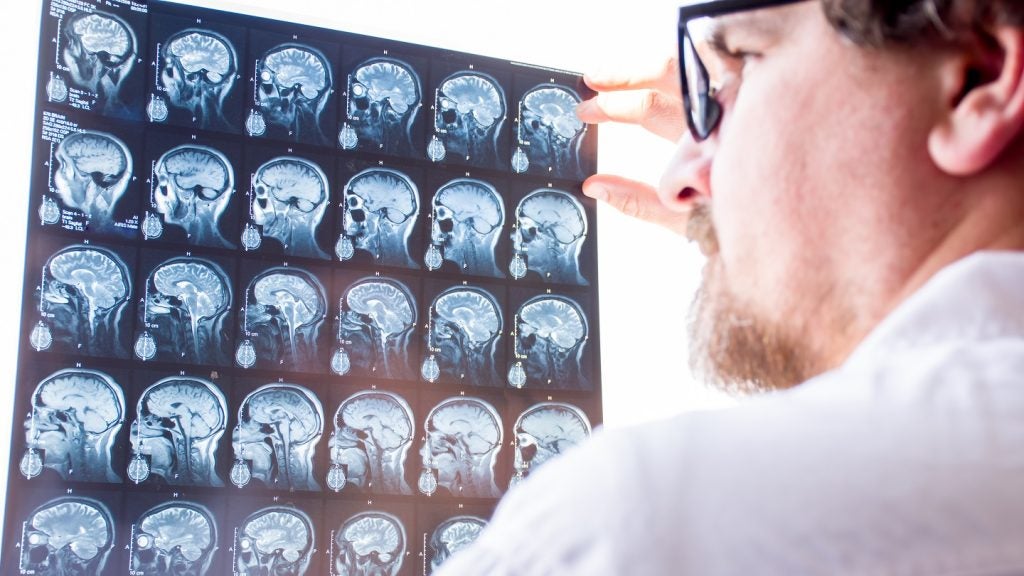
Imcyse has concluded the enrolment of subjects and initial dosing in the Phase I part of its adaptive Phase I/II IMCY-MS-001 clinical trial of IMCY-0141 to treat relapsing-remitting multiple sclerosis (RRMS).
The open-label, dose-escalation part will evaluate the safety of three Imotope IMCY-0141 doses in adult patients with RRMS.
Imcyse CEO Denis Bedoret said: “IMCY-0141 is now the second Imotope from Imcyse's proprietary platform to progress to the next clinical stage, demonstrating our commitment to finding new therapies for patients suffering from severe, chronic autoimmune diseases.”
Upon the completion of the Phase I portion, Imcyse plans to initiate a Phase II expansion arm of the study to evaluate immune responses and disease marker activity.
This trial will also be used for optimising the dose for further registration studies.
Imcyse CCDO Jean Van Rampelbergh said: “The pre-specified Independent Data Monitoring Committee [IDMC] analyses to date have found no safety concerns, and we expect the final outcome of the IDMC's review of the data from the third and highest dose level from our Phase I trial by the end of this year.
“The results from the Phase I portion of the trial will allow further refining of the Phase II protocol design and selection of the most appropriate doses to be evaluated.
“We look forward to continuing to explore the role of IMCY-0141 in the treatment of multiple sclerosis, which, if applied early on in the course of the disease, could slow down or even halt its progression, leading to a potentially curative approach.”
Imcyse is also developing a pipeline of Imotopes to treat various autoimmune diseases in large and rare disease indications.