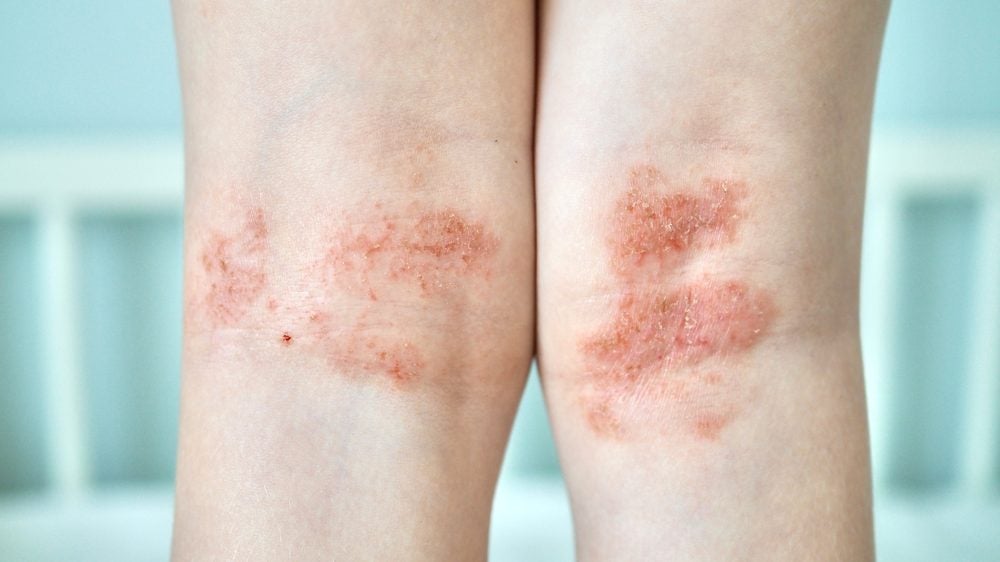
Incyte has reported positive long-term extension (LTE) data from the TruE-V trial. The study assessed the safety and efficacy of Opzelura (ruxolitinib)as a treatment for nonsegmental vitiligo in patients who had previously seen no improvement from the treatment after 24 weeks of use.
More than half of the patients who initially had no response to treatment with Opzelura at week 24 achieved a 75% improvement in facial repigmentation at week 104. Fifty percent of the patients, who had little to no response to treatment at week 24, saw an improvement in body repigmentation at week 104,
The TRuE-V clinical trial programme is made up of two Phase III studies, TRuE-V1 (NCT04052425) and TRuE-V2 (NCT04057573). Each study enrolled approximately 300 patients above the age of twelve years old with nonsegmental vitiligo.
Dr Albert Wolkerstorfer from the Netherlands Institute for pigment disorders said: “These data highlight the importance of continuing treatment with Opzelura in patients with vitiligo, even when minimal or no repigmentation is achieved after six months of treatment.”
Topline positive Phase III results were reported in May 2021 for the original 24 week TRuE-V1 and TRuE-V2 programmes. The data showed that both trials met their primary endpoints, that measured the proportion of patients with facial vitiligo severity index score 75 (F-VASI75). F-VASI75 is determined as a 75% improvement from baseline to week 24.
However, in March 2022, the US Food and Drug Administration (FDA) requested additional information from the trials, describing the lack of this information as a “major amendment” to Incyte’s application for vitiligo. The FDA approved the cream for the condition in July 2022.
Opzelura cream 15mg/g is approved in the EU for the treatment of non-segmental vitiligo with facial pigmentation in patients over twelve years old.
According to GlobalData, Opzelura sales are expected to rise from $129m in 2022 to $1.4bn by 2029. GlobalData is the parent company of Clinical Trials Arena.