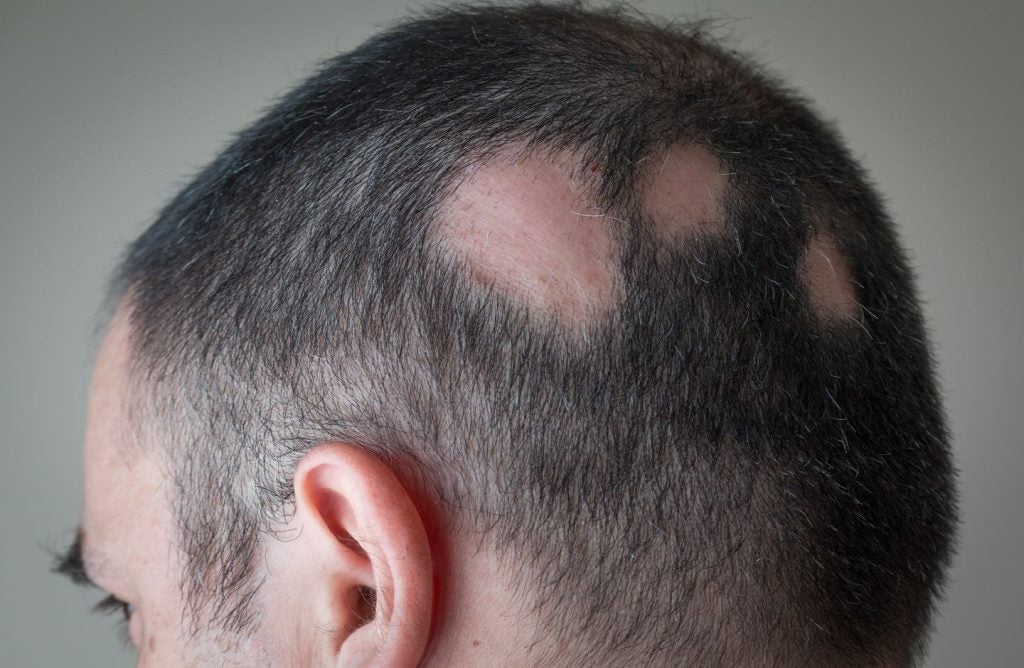
Inmagene Biopharmaceuticals has dosed the first subject in a Phase IIa trial of IMG-007, an anti-OX40 monoclonal antibody (mAb) with an extended half-life, to treat alopecia areata (AA).
The global multicentre trial will assess the efficacy, safety, biomarkers, and pharmacokinetics of IMG-007 in AA patients with 50% or greater scalp hair loss.
The humanised IgG1 mAb particularly attaches to the OX40 receptor as well as designed to potently block the signalling between OX40 and its ligand.
IMG-007's extended half-life enables less frequent dosing while its silenced antibody-dependent cell-mediated cytotoxicity (ADCC) function cuts safety risks.
It has shown a favourable safety profile, without any pyrexia or chill reports, in a Phase I study in healthy adult participants.
A single IMG-007 treatment at projected therapeutic dose levels maintained the desired exposure for a duration of 12 to 18 weeks, potentially enabling IMG-007 to be administered every 12 weeks.
It is also being assessed in a global Phase IIa study in adults with atopic dermatitis (AD) with an interim data readout anticipated in the second quarter of next year.
Inmagene CEO Jonathan Wang said: “Alopecia areata is a devastating disease which affects approximately 147 million people globally. Currently, there are limited treatment options and no approved biologics for AA.
“IMG-007 could potentially provide a safe and effective biologic therapy with once every 12 weeks dosing regimen for AA patients.”
AA is a chronic autoimmune condition causing hair loss without scarring, which can affect the scalp, face, and body.