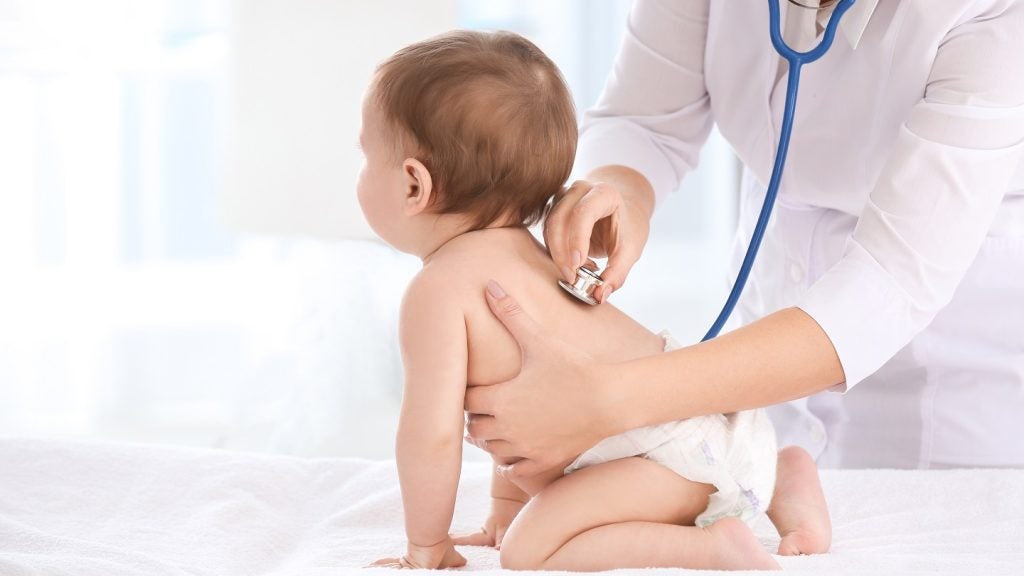
Inozyme Pharma has dosed the first infant in the Phase Ib ENERGY-1 clinical trial of INZ-701 enzyme replacement therapy to treat ENPP1 deficiency.
The open-label, single-arm study is designed to evaluate the pharmacokinetics (PK), pharmacodynamics (PD), tolerability, and safety of the therapy in infants with ENPP1 deficiency.
It intends to enrol up to eight infants aged between one and 12 months from various sites in Europe and the US.
Infants will be administered INZ-701 subcutaneously for 52 weeks. In an extension period beyond 52 weeks, patients may also receive INZ-701 dosages.
Doses of the therapy range from 0.2mg/kg once weekly to 0.6mg/kg twice a week, and may further increase based on the PK/PD and safety data of the INZ-701 study.
Evaluation of exploratory biomarkers, cardiac function, functional performance, development, survival, growth, and plasma pyrophosphate (PPi) levels are additional outcome measures of the study.
Inozyme Pharma senior vice-president and chief medical officer Kurt Gunter said: “Initiation of the ENERGY-1 trial in infants is an important milestone, as we continue to advance INZ-701 with the goal of improving the lives of patients with ENPP1 deficiency across all age groups.
“We are committed to a global programme to identify and treat all newborns with this condition.”
INZ-701 is a recombinant Fc fusion protein and is in development for treating rare disorders of the skeleton, soft tissue, and vasculature.
It is currently being evaluated in Phase I/II clinical trials to treat ABCC6 deficiency, along with ENPP1 deficiency.