Johnson & Johnson (J&J) has shared data from Cohort B of the Phase IIIb VISIBLE study, indicating that TREMFYA (guselkumab) significantly aids in clearing moderate to severe scalp psoriasis (PsO) in people of colour, according to a company release.
This marks the first prospective, large-scale, randomised-controlled trial to evaluate the drug's efficacy across all skin tones, focusing on objective clearance and treatment outcomes.
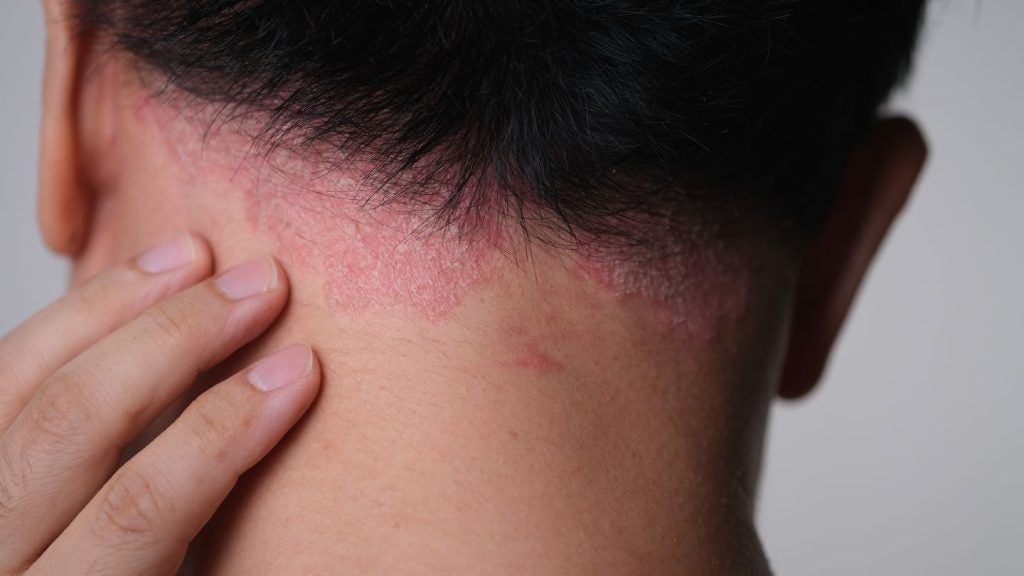
Cohort B of the trial included 108 participants with scalp predominant moderate-to-severe PsO.
According to the findings, after 16 weeks and three doses of TREMFYA, patients reported substantial improvements in scalp itch and health-related quality of life outcomes (HRQoL), including reduced pigmentation post-inflammation, against placebo.
These subjects also reported mild effects from skin discolouration on their HRQoL.
Johnson & Johnson Innovative Medicine, Immunology and Medical Affairs vice-president Jennifer Davidson said: “Scalp psoriasis can be particularly burdensome to patients as it often impacts more visible areas of the body, including the hairline, forehead, neck, and around the ears, triggering feelings of self-consciousness that limit many people’s lifestyle choices.
“The VISIBLE study suggests that people of colour continue to experience undertreatment, with many participants not receiving a biologic option prior to this trial enrolment.
“The insights from the study aim to empower diverse patients with moderate to severe plaque and scalp psoriasis to learn more about their treatment options and initiate informed discussions with their providers.”
The results build on data from Cohort A of the VISIBLE study, which were released in October last year.
The VISIBLE trial enrolled patients with skin predominant PsO. This cohort also offered further safety and efficacy data on TREMFYA across different skin tones.
TREMFYA is currently approved for use in the US for treating adults with moderate to severe plaque PsO who have received systemic therapy or phototherapy, as well as adult patients with active psoriatic arthritis (PsA).