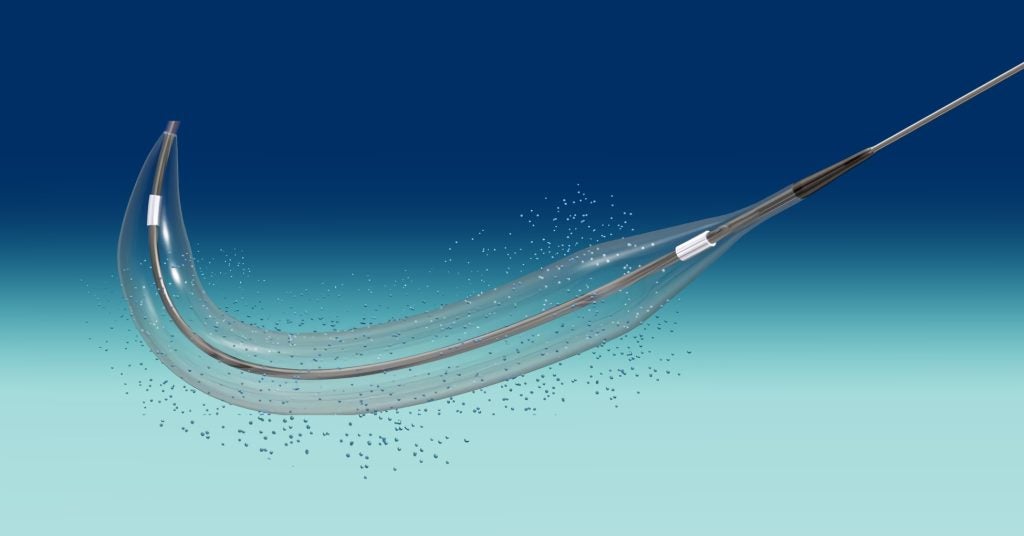
The final patient has been enrolled in MedAlliance’s SAVE trial investigating the safety and feasibility of its drug-eluting balloon for the treatment of failed arteriovenous fistula in renal dialysis patients.
The Selution Sustained Limus Release (SLR) device, which received investigational device exemption by the US Food and Drug Administration (FDA) in 2022, is a sirolimus-eluting balloon being tested in a prospective, multi-centre, single-blinded, randomised trial (NCT04327609).
The Swiss medtech company has enrolled 84 patients who have been split into a Selution SVR treatment group and a control group (with standard high-pressure balloon angioplasty).
The study endpoint is primary patency at six months with angiographic follow-up. The company will also assess freedom from serious adverse events at 30 days.
The device releases sirolimus, an anti-restenotic drug, via micro reservoirs applied to the surface of the balloon. The reservoirs, which contain the drug with a biodegradable polymer, can release the drug for up to 90 days. The device is already commercially available in Europe, Asia and the Middle East and the Americas. Data from the SAVE trial will be used to achieve approval for AVF indication in North America.
Dr Konstantinos Katsanos, principal investigator of the trial said: “This is the first prospective randomised trial of a sirolimus-coated balloon in AVF patients with angiographic follow-up. Furthermore, we have also measured fistula volume flow rates, which is another key index of failing or maintained fistula function.”
In March 2023, MedAlliance announced it had enrolled more than 1,000 patients in a trial investigating the device in coronary artery disease treatment.
A market model by GlobalData estimates that the percutaneous transluminal coronary angioplasty (PTCA) balloon catheter market will be worth $1.8bn by 2033. Drug-coated balloons will contribute nearly $300m to the market.