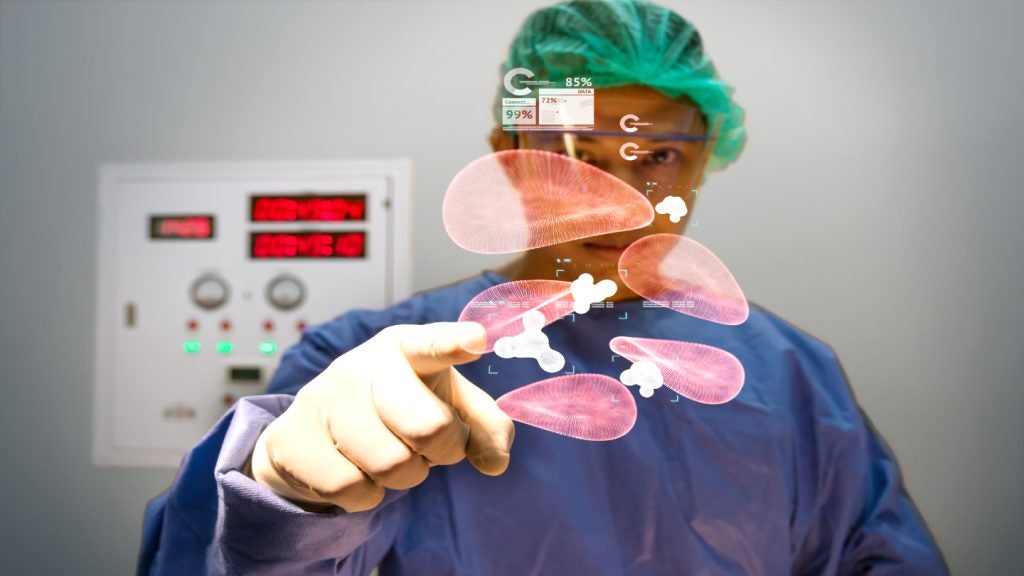
The Cleveland-based augmented reality (AR) firm, MediView XR, has announced the results of a trial studying its XR90 surgical visualization and navigation system, finding it accurate enough to carry out procedures with clinical precision.
The XR90 system is a US Food and Drug Administration (FDA) 510(k) cleared device currently in clinical usefor ultrasound-guided needle-based procedures for soft tissue and bone.
MediView said that its XR90 system gives physicians 3D X-ray vision during procedures, giving clinicians the ability to see a patient's internal anatomy in 3D, including bone, tissue, organs, and vasculature.
For this study, published in the Journal of Medical Imaging, researchers sought to quantify the targeting and image fusion registration accuracy of MediView's AR image guidance platform for needle-based procedures. Researchers used cadavers implanted with targets mimicking cancerous tumours inside the liver. Researchers carried out 36 procedures performed on four cadavers.
Researchers evaluated the accuracy of the system using two methods, Target registration error (TRE), a measurement of the main system error and image fusion registration error (IFRE), a measurement of multimodal registration error between different imaging modalities such as CT and ultrasound. The results showed a mean TRE of 2.3mm and a mean IFRE of 4.4mm.
The study results detail how the accuracy metrics reported by researchers showed that the AR needle guidance system can be used to reach targets with the precise degree of accuracy required for clinical applications when used as an adjunct to standard-of-care imaging.
GlobalData’s Medical Device Intelligence Centre found that the overall global market for AR was estimated at a value of $7bn in 2020, with that figure expected to grow to $152bn by 2030, growing at a compound annual growth rate (CAGR) of 36%.
Charles Martin, the study investigator at the Cleveland Clinic, said: “Having a mean TRE of under 5mm establishes that MediView's XR90 system has the potential to be used for targeting within the borders of the smallest treatable tumours, which is oftentimes observed during clinical practice.
“The ability of this AR-based navigational guidance system to reliably facilitate needle trajectory planning and guidance for percutaneous procedures could potentially facilitate better clinical performance." Elsewhere in the field of AR and surgery, the first paediatric deformity surgery using Novarad’s VisAR, an augmented reality surgical navigation system has been performed at Washington University School of Medicine in St Louis. US tech giant Apple has also entered the VR market, with its extended reality (XR) technology set to make an impact in the medical sector most of all.