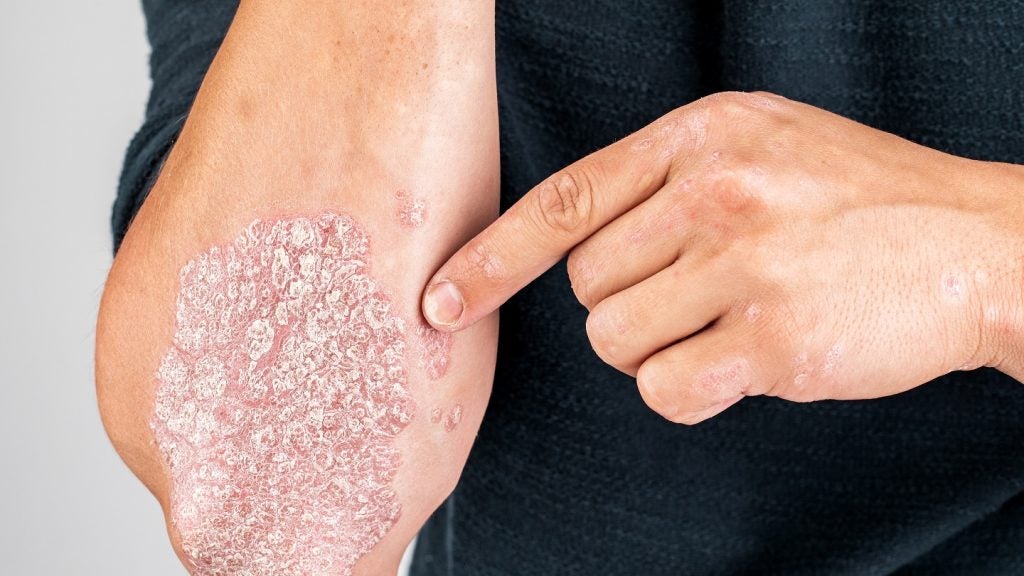
Meiji Seika Pharma has reported that its Phase II study of ME3183 for the treatment of plaque psoriasis met the primary endpoint based on Psoriasis Area Severity Index (PASI)-75 score at week 16 from baseline.
Conducted in the US and Canada, the trial was supervised by Meiji Pharma USA, a subsidiary of Meiji.
The study has assessed the safety, tolerability, and efficacy of the selective phosphodiesterase-4 (PDE4) inhibitor ME3183 in patients with moderate-to-severe plaque psoriasis.
It enrolled 132 patients who received ME3183 orally once or twice daily for 16 weeks.
Compared to the placebo group, a significant greater proportion of PASI-75 was achieved in ME3183 treatment groups.
Early PASI improvement after administration was also observed in the treatment groups.
Greater anti-inflammatory effect and nearly 30-fold greater inhibitory effect on TNF-α production was also observed in non-clinical studies of ME3183 in comparison to existing orally-available PDE4 inhibitor for psoriasis.
The distribution of ME3183 in the brain was also sufficiently low.
Meiji is also engaged in developing safe treatments for other autoimmune diseases along with psoriasis.
Last December, Meiji Seika Pharma initiated a Phase III trial of ARCT-154, a messenger ribonucleic acid (mRNA) vaccine for the treatment of Covid-19, in Japan.
Developed by Arcturus Therapeutics, ARCT-154 is claimed to be effective against SARS-CoV-2 viral variants, including the Omicron variant.