The US National Institutes of Health (NIH) has reported that etidronate, an existing drug typically used to treat bone diseases, demonstrated potential in reducing the progression of Arterial Calcification due to Deficiency of CD73 (ACDC), a rare genetic condition.
Supported by the NIH unit National Heart, Lung, and Blood Institute (NHLBI), the trial was designed to assess the safety and effectiveness of etidronate in treating calcification of the arteries and impaired blood flow in the legs of ACDC patients.
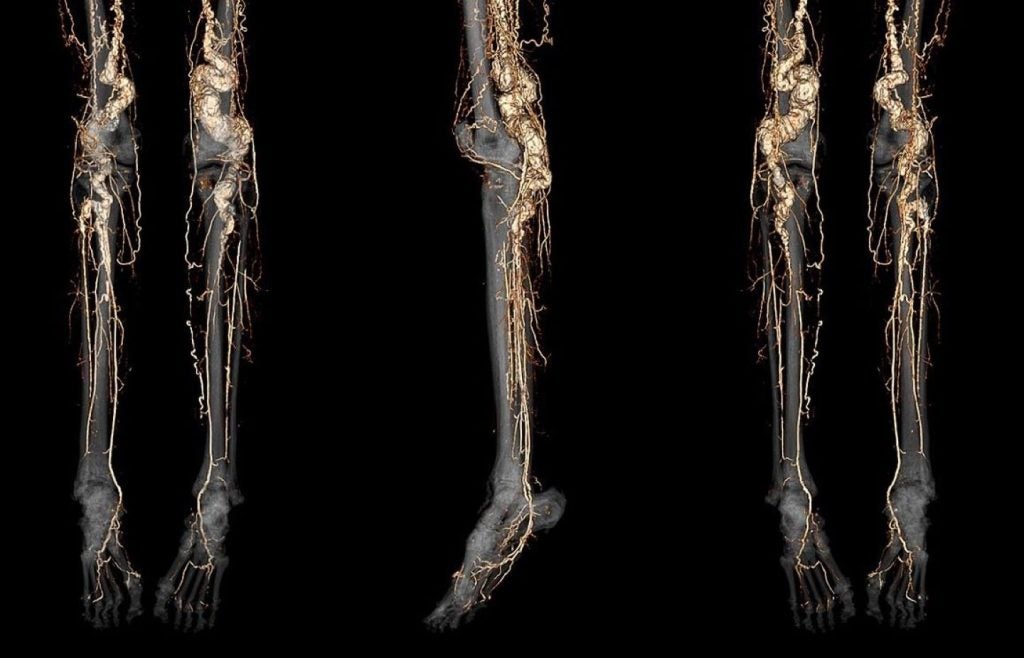
In the study, seven ACDC patients received treatment with etidronate, which was given orally for 14 days every three months over three years.
Researchers assessed calcium deposits with CT scans and tested flow of blood with the help of ankle brachial index at the beginning of the study and during yearly follow-ups.
The trial results indicated that the etidronate treatment was safe without any adverse side effects observed.
Furthermore, the drug showed to slow the progression of fresh calcium deposits in the leg blood vessels and the blood flow inhibition.
However, the treatment did not reverse existing calcium deposits or significantly improve blood flow.
Patients also reported symptom improvements, such as reduced pain and motion impairment.
The implications of this study are far-reaching, as the findings could help develop new treatments for ACDC and inform larger clinical trials targeting the disease.
Additionally, the research could provide insights into other conditions characterised by excessive calcium buildup in the arteries, such as peripheral artery disease and atherosclerosis, the NIH noted.
ACDC, which currently has no known treatment, predominantly affects the leg arteries, leading to painful and difficult walking.
It can also impact the hand joints, causing pain and deformities. In extreme cases, ACDC can result in limb loss.