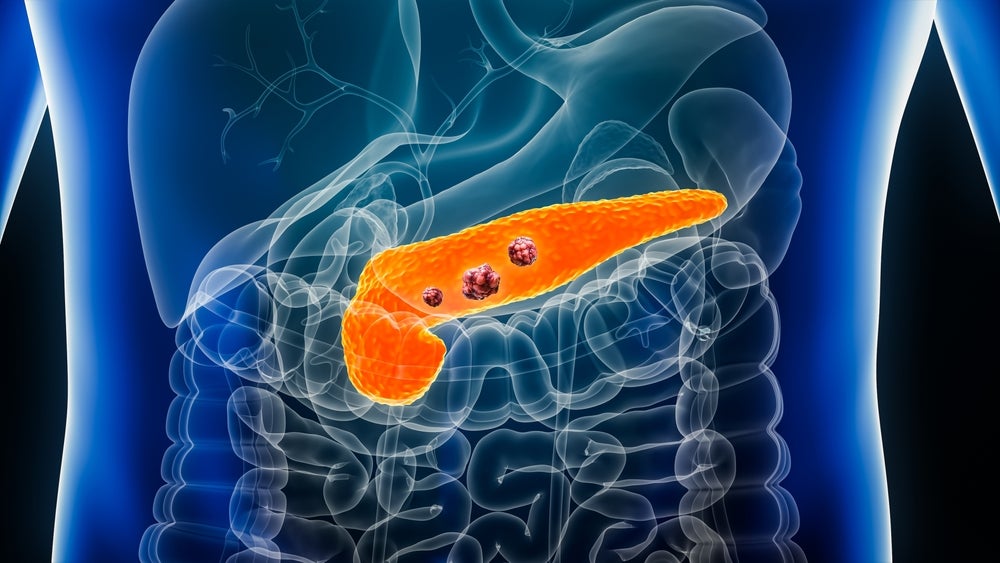
Novartis has announced that Lutathera along with octreotide long-acting release (LAR) has reduced the risk of disease progression or death in patients with advanced gastroenteropancreatic neuroendocrine tumours (GEP-NETs) in a trial.
This was demonstrated in the Phase III NETTER-2 clinical trial, where the combination therapy extended median progression-free survival (PFS) to 22.8 months compared to 8.5 months with high-dose octreotide LAR alone.
The trial compared Lutathera plus octreotide LAR to high-dose octreotide in patients with advanced GEP-NETs. It included patients diagnosed with somatostatin receptor-positive (SSTR)-positive advanced GEP-NETs within six months before enrolment.
NETTER-2 is a landmark study, being the first to show positive results for a radioligand therapy (RLT) in a first-line setting.
During the 2024 American Society of Clinical Oncology (ASCO) Gastrointestinal (GI) Cancers Symposium, data revealed a 72% reduction in the risk of disease progression or death when using Lutathera as a first-line therapy.
The safety profile of the therapy was consistent with previous findings, with most patients tolerating the full course of treatment.
The NETTER-2 trial continues to assess secondary endpoints, including long-term safety and overall survival. This ongoing research is crucial for improving treatment strategies for patients with high-proliferation rate tumours.
Lutathera is already approved in the US, Europe, and Japan for certain types of GEP-NETs.
Novartis oncology development global head Jeff Legos said: “This is the first positive Phase III trial of a radioligand therapy in the first-line setting, and the overall efficacy and safety results are amongst the most clinically relevant observed to date in this kind of advanced cancer, addressing a significant unmet need for patients with newly diagnosed advanced GEP-NETs.
“The positive results are a significant advancement and further reaffirm our strategy to research and develop radioligand therapies in earlier lines of treatment or stages of disease to improve outcomes for patients.”