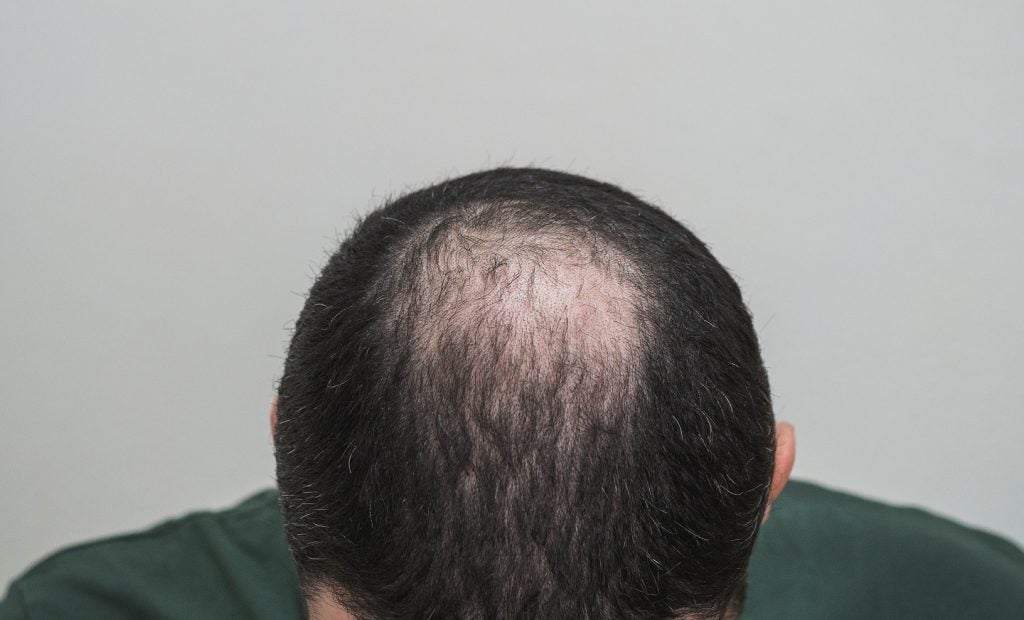
OliX Pharmaceuticals has administered the first patient with its RNAi therapeutic, OLX72021, in a Phase I trial to treat androgenic alopecia, also called male-pattern baldness.
The administration was carried out at a designated clinical study facility in Australia.
After securing approval for the trial from Australia’s Bellberry Human Research Ethics Committee (Bellberry HREC) this March, OliX started patient enrolment.
The trial’s primary objective is to examine the safety, tolerability, and pharmacokinetics of OLX72021 given through a single intradermal injection.
The trial splits up to 30 healthy males with androgenic alopecia into five cohorts, with eight weeks follow-up monitoring following intradermal injection of OLX72021 in several dosages, or a placebo, in six alopecia areas near the patients’ crown.
OLX72021 has the capability to suppress the hormone activity that leads to androgenic alopecia by minimising the androgen receptor (AR) expression.
In pre-clinical studies, OLX72021 showed long-term efficacy. The therapy is anticipated to reduce the inconvenience of current hair loss treatments that need frequent injection or administration.
As OLX72021 is topically injected into the scalp and degraded quickly when exposed to the blood after maintaining high concentration only in subcutaneous hair loss areas, it may not cause the same side effects and limitations, including sexual dysfunction or depression, as current hair loss treatments.
OliX received a patent for the therapy in 2021 in the US.
Following the confirmation of safety of OLX72021 in human bodies in the Phase I trial in Australia, the company plans to introduce new hair loss cosmeceuticals in the future.