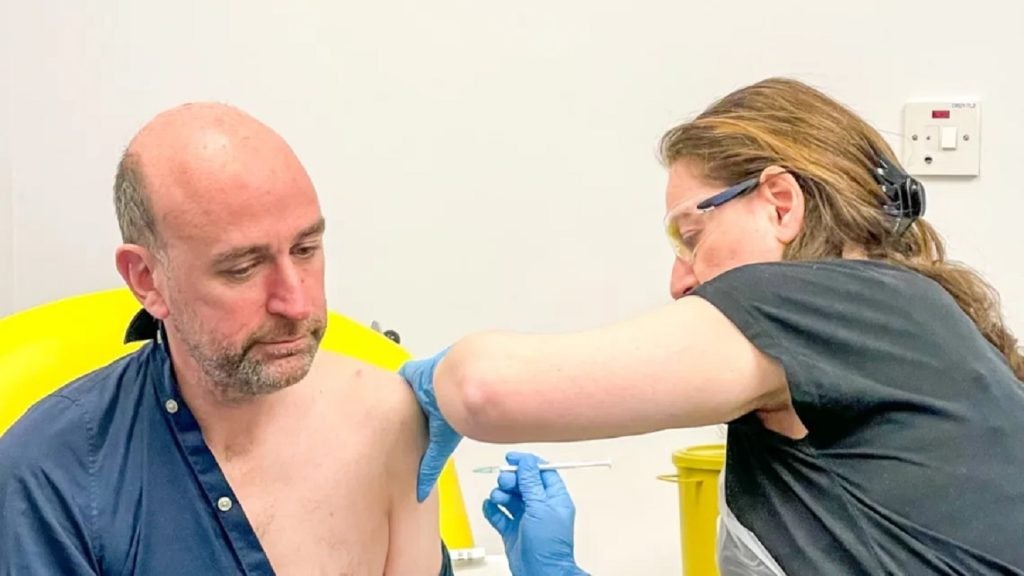
The University of Oxford in the UK has initiated a clinical trial to evaluate a vaccine designed to combat the lethal Nipah virus.
This marks the first-in-human trial for the ChAdOx1 NipahB vaccine, a product being developed by the University's Pandemic Sciences Institute.
The trial will involve 51 individuals between the ages of 18 and 55 and is led by the Oxford Vaccine Group, part of the Department for Paediatrics.
The Coalition for Epidemic Preparedness Innovations (CEPI) is providing financial support for the trial.
Centre for Clinical Tropical Medicine and Global Health infectious diseases professor and the trial’s principal investigator Brian Angus said: “Nipah virus was first identified in 1998, and yet 25 years on the global health community still has no approved vaccines or treatments for this devastating disease. Due to the high mortality rate and the nature of Nipah virus transmission, the disease is identified as a priority pandemic pathogen.
“This vaccine trial is an important milestone in identifying a solution that could prevent local outbreaks from occurring, while also helping the world prepare for a future global pandemic.”
Nipah virus is known for its high fatality rate, with approximately 75% of infections resulting in death.
The World Health Organization has identified Nipah as a priority disease that necessitates urgent research due to its potential for severe outbreaks.
The current trial is expected to span 18 months, with subsequent studies planned in regions affected by the Nipah virus.
CEPI Vaccine Research & Development acting executive director Dr In-Kyu Yoon said: “Nipah has epidemic potential, with its fruit bat hosts found in areas home to over two billion people. This trial is a step forward in efforts to build a suite of tools to protect against this killer virus. Knowledge gained could also inform the development of other Paramyxovirus countermeasures.”
Last year, Oxford University Hospitals NHS Foundation Trust started a clinical trial of a messenger ribonucleic acid (mRNA) vaccine to treat patients with head and neck cancer.