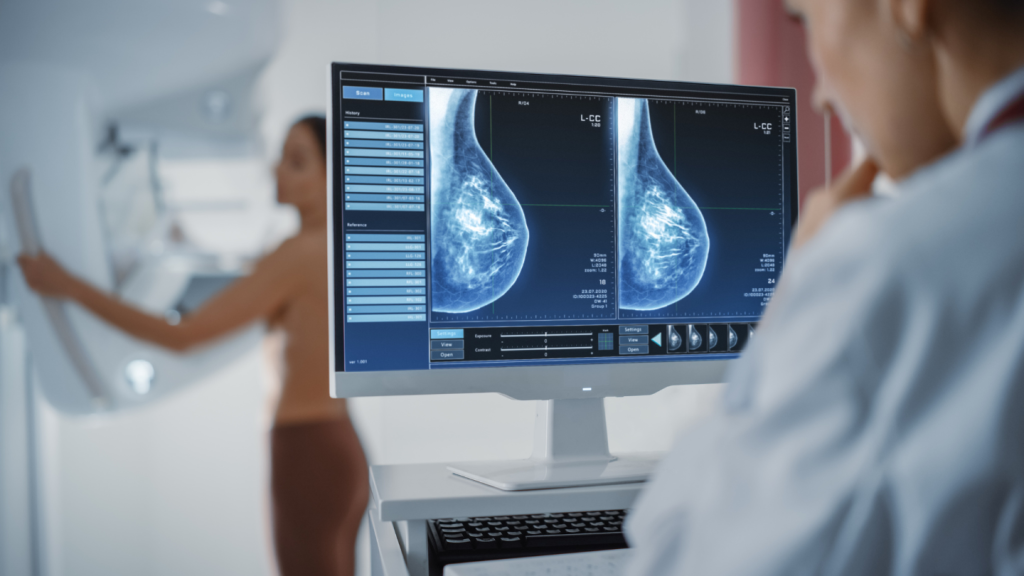
Seagen’s Phase III HER2CLIMB-02 trial of tucatinib and ado-trastuzumab emtansine met its primary endpoint of progression-free survival (PFS).
The global, multicentre, randomised, double-blind, placebo-controlled, Phase III clinical trial (NCT03975647) began enrolment in 2019.
The primary endpoint of the trial of PFS has been met however data for the secondary endpoint of overall survival are not yet mature. More patients in the combination arm discontinued treatment due to adverse events but no new safety signals emerged.
Investigators used tucatinib, which is branded as Tukysa, in combination with the antibody-drug conjugate ado-trastuzumab emtansine, which is marketed as Kadcyla, in patients with unresectable locally advanced or metastatic human epidermal growth factor receptor 2-positive (HER2-positive) breast cancer. Patients in the trial had received previous treatment with taxane and trastuzumab.
Tukysa is an orally administered tyrosine kinase inhibitor of the HER2 protein and is approved in more than 40 countries. Merck (MSD) has exclusive rights to commercialise Tukysa in regions outside of the US, Canada and Europe. Seagen is also conducting a study of Tukysa with first-line maintenance with trastuzumab and pertuzumab as part of its robust development plan for the drug.
Kadcyla is an antibody-drug conjugate consisting of the humanised monoclonal antibody trastuzumab covalently linked to the cytotoxic agent DM1. It was approved for use by the US Food and Drug Administration (FDA) in 2013 and was developed by Genentech.
Results of the HER2CLIMB-02 trial
Seagen's research and development president and chief medical officer Roger Dansey said: “We are encouraged by these results for Tukysa in combination with Kadcyla in metastatic HER2-positive breast cancer, including in patients with brain metastases.
"We plan to present the HER2CLIMB-02 data at an upcoming medical meeting and discuss the results with the FDA.”
Adverse and serious reactions
In the HER2CLIMB parent study, serious adverse reactions occurred in 26% of patients the most common being diarrhoea, vomiting, nausea, abdominal pain and seizure. Fatal adverse reactions occurred in 2% of patients who received Tukysa, including sudden death, sepsis, dehydration, and cardiogenic shock.
Of patients receiving Tukysa, adverse reactions led to treatment discontinuation in 6% of patients and a dose reduction in 21% of patients. The most common adverse reactions in patients were diarrhoea, palmar-plantar erythrodysesthesia, nausea, hepatotoxicity, vomiting, stomatitis, decreased appetite, anaemia and rash.