US-based biopharma company Sirnaomics is preparing for a registrational Phase III trial investigating STP705 in squamous cell carcinoma in situ (isSCC).
The company announced plans after concluding the end of the Phase II trial (EOP2) meeting with the US Food and Drug Administration (FDA).
Sirnaomics stated that it is well-positioned to advance STP705 into a confirmatory clinical trial for the treatment of isSCC. This year, the company is proceeding with a single-dosage study investigating a sub-group of subjects in a prospective large Phase III trial.
The positive results from the sub-group cohort will provide a basis for completing the registrational Phase III trial, although the company did not disclose the estimated completion date.
STP705 is a small interfering RNA (siRNA) therapeutic that acts by targeting transforming growth factor-beta 1 (TGF beta 1) and cyclooxygenase-2 (COX-2).
The therapy is also being studied in basal cell carcinoma (BCC). Sirnaomics noted that this would be the next candidate to move into a late-stage development pending the FDA’s review.
The drug has been studied in more than 100 participants with isSCC or BCC. Based on prior clinical studies in both cancer types, the safety profile is favourable, with no grade 3 or higher adverse events.
Phase IIb data
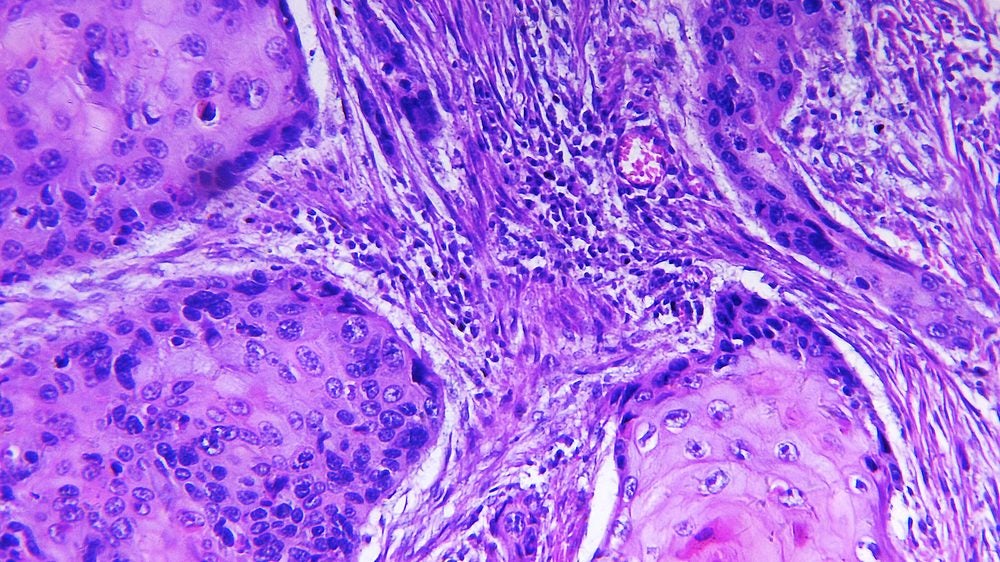
Sirnaomics reported positive interim Phase IIb trial data in December 2022. The data analysis from part one of the trial showed that 78% of patients treated with STP705 achieved histological clearance.
Amongst the three treatment cohorts, the low-dose treatment group achieved 89% histological clearance while the mid-dose and high-dose groups achieved 75% and 73%, respectively. The placebo cohort achieved 58%.
The Phase IIb trial (NCT04844983) is a two-part double-blind study. The first part recruited 44 patients while part two was estimated to include another 60 subjects, according to the Sirnaomics’ announcement in December 2022. Patients received STP705 or placebo injection once a week for six weeks.
The primary endpoint measured the proportion of participants with histological clearance of treated isSCC lesions while the secondary endpoint measured the change in size of the treated lesions.
Other development announcements
Earlier this month, Sirnaomics reported interim results from a Phase I trial (NCT05422378) investigating STP705 for fat reduction in adults undergoing abdominoplasty.
The interim efficacy results were examined for six patients about to undergo abdominoplasty. The preliminary data indicated that STP705 was safe as a treatment for unwanted fat and showed signs of efficacy.
In addition, Sirnaomics dosed the first patient in a Phase I trial investigating STP122G, an RNAi drug candidate for anticoagulant treatment. The trial intends to enrol 40 healthy volunteers.