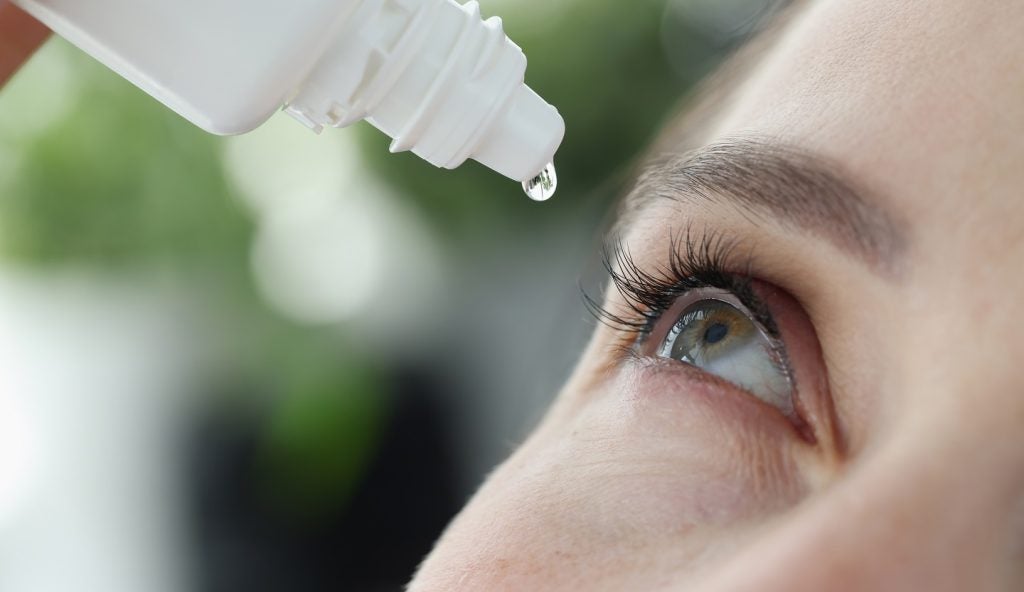
US-based biopharmaceutical company Skye Bioscience has finished enrolling patients in its Phase I trial of SBI-100 Ophthalmic Emulsion (OE), a cannabinoid receptor type 1 (CB1R) agonist that is given topically onto the eye.
A total of 48 healthy patients have been enrolled in the trial.
Skye Bioscience has also finished dosing subjects in the third and final cohort of the trial’s multiple ascending dose (MAD) arm.
The company will provide an update after a safety review committee has evaluated the last cohort’s data over the coming weeks.
Skye Bioscience plans to deliver a preliminary data report in the third quarter of this year.
The Phase I trial aims to assess SBI-100 OE's tolerability, safety and pharmacokinetics with multiple dosing regimens, as well as calculate changes in intraocular pressure.
It is being conducted as a randomised, double-masked, placebo-controlled study.
Patients in the trial have been randomised into a single ascending dose (SAD) arm or MAD arm.
Both arms have three cohorts, which have eight subjects each. In each cohort, six patients have been treated with SBI-100 OE and two with placebo.
SBI-100 OE was given topically in one eye at increasing doses of 0.5%, 1% and 2% in the two arms' individual cohorts.
Subjects in the MAD arm received one dose each morning and evening for five days. They were supervised at the clinical research unit for seven days, including the five days of dosing.
The trial is currently being carried out in Adelaide, Australia.
SBI-100 OE was found to be well-tolerated in the completed SAD arm and initial two cohorts of the MAD arm.
There were no drug-related serious adverse events in the trial, but some mild and moderate adverse events were reported.
These adverse events are consistent with eye treatments that are applied topically.