Abaloparatide-SC is an injectable formulation of abaloparatide developed by Radius Health for the treatment of osteoporosis in post-menopausal women.
Radius Health has submitted the marketing authorisation application (MAA) for Abaloparatide-SC in Europe on 17 November 2015. The European Medical Agency (EMA) has accepted the application for review on 4 December 2015 and the same is currently under review.
The new drug application (NDA) for abaloparatide-SC was submitted to the US Food and Drug Administration (FDA) on 30 March 2016.
Post-menopausal osteoporosis
The cessation of menstrual cycle, menopause occurs in women aged between 45 and 55. The oestrogen levels are affected during the menopause, which increases the risk of developing post-menopausal osteoporosis (Type 1 osteoporosis).
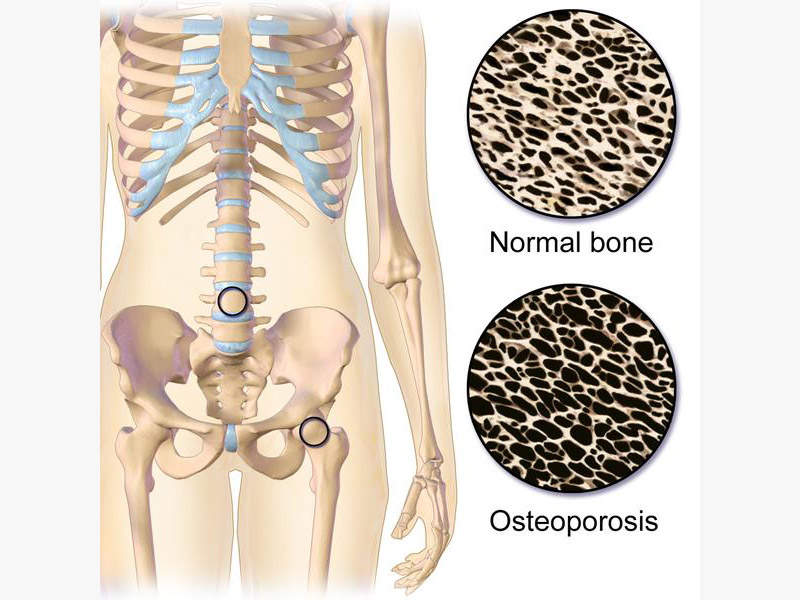
Osteoporosis is a bone disease, in which the bones become thin, fragile and break easily. It usually affects all the bones in the body but commonly occur in hip, wrist and spine. More than 200 million people worldwide suffer from osteoporosis and approximately two million osteoporosis factures are estimated to occur in the US alone.
Post-menopausal osteoporosis makes skeletal bones more sensitive to the parathyroid hormone, which in turn causes calcium resorption from bones causing loss of bone mass and leading to fractures.
The prevalence of post-menopausal osteoporosis in the US is found to be in 15.4% of women older than 50 years and roughly 34.9 % of women older than 80 years.
Abaloparatide-SC’s mechanism of action
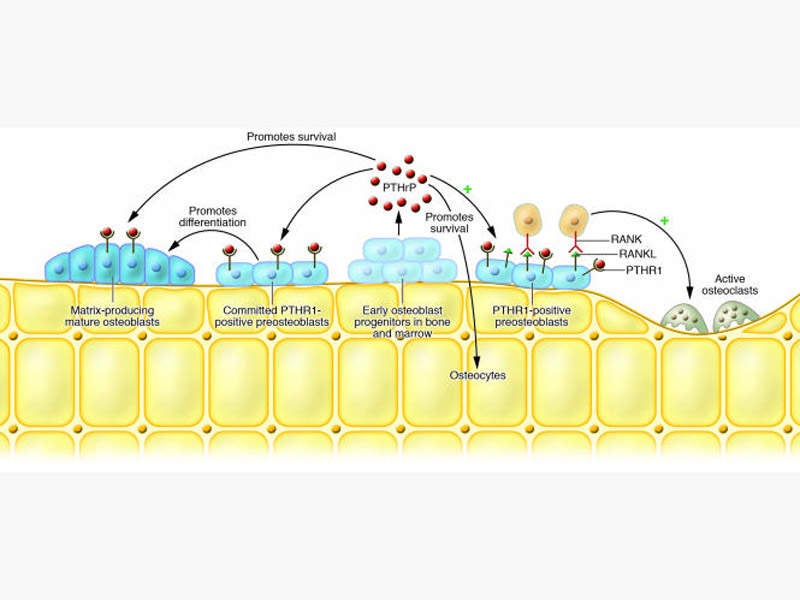
Abolaparatide-SC is a synthetic peptide analogue of human parathyroid hormone-related protein (hPTHrP), which interacts with parathyroid receptor 1 and exhibits anabolic actions to regulate the bone formation.
Parathyroid hormone-related protein plays an important role in the formation of the skeleton and rebuilds the bone without inducing any side effects such as hypercalcaemia.
If approved by the FDA and the EMA, abolaparatide-SC will be the first new bone anabolic treatment for post-menopausal osteoporosis in the US since 2002 and in Europe since 2003.
Clinical trials on Abolaparatide-SC
The NDA for abolaparatide-SC was submitted to the FDA based on the results from two phase III clinical trials namely ACTIVE and ACTIVExtend.
The ACTIVE trial is a randomised, double-blind, placebo-controlled, comparative phase 3 multi-centre study designed to evaluate the efficacy and safety of abolaparatide-SC 80µg injection in preventing fractures in the women with severe post-menopausal osteoporosis.
The study enrolled 2,463 patients suffering from severe osteoporosis with high risk of fractures. The subjects were randomised to either placebo arm or abaloparatide-SC arm or teriparatide arm.
The drug achieved both primary and secondary end points of significant reduction of vertebral fractures and non-vertebral fractures and clinical factures when compared to placebo.
The ACTIVExtend trial is an extension study to evaluate 24 months of standard care of the osteoporosis management after the completion of the ACTIVE trial. It is also a randomised, open-label, placebo-controlled and comparative phase 3 trial.
The study enrolled 1,139 patients in the first six months of the trial and the patients were treated on alendronate therapy for osteoporosis management. The trial has met all the primary and secondary endpoints of reduction of vertebral fractures, non-vertebral fractures, clinical and other osteoporotic fractures.
The most common adverse events reported were arthralgia, dyspepsia, upper respiratory infection, urinary tract infection and bone pain.