Roche Pharmaceuticals’ Actemra (tocilizumab) was approved as a rheumatoid arthritis treatment in the EU in January 2009 and the US in January 2010. The drug is marketed under the trade name RoACTEMRA in Europe.
A combination of Actemra and methotrexate can be used for the treatment of adult patients with moderate to severe rheumatoid arthritis who failed to respond to, or were intolerant to, previous therapy with one or more tumour necrosis factor antagonists or disease-modifying, anti-rheumatic drugs.
Actemra is therefore appropriate where continued methotrexate treatment is not beneficial or to patients who are methotrexate intolerant. It can be given as monotherapy where Actemra is the interleukin-6 (IL-6) receptor-inhibiting monoclonal antibody.
Roche conducted five multinational phase III studies, which demonstrated that Actemra reduced the signs and symptoms of rheumatoid arthritis compared with other therapies when given as either a monotherapy or in combination with other disease-modifying, anti-rheumatic drugs.
One month after the launch of the drug in the US, BioTrends Research Group carried out a study on its market uptake. The results showed that 38% of rheumatologists had started prescribing Actemra to their patients.
In January 2011, the US Food and Drug Administration (FDA) approved an extension label for Actemra based on the results of the LITHE study. The drug can be used in combination with methotrexate for the inhibition and slowing of structural joint damage, improvement of physical function, and major clinical response in adults.
Actemra also received approval from the FDA in April 2011 for the treatment of Systemic Juvenile Idiopathic Arthritis (SJIA) a rare form of arthritis found in children. It is the first drug to be approved by the FDA for the treatment of SJIA. The approval was based on the results of the phase III TENDER trial.
Actemra approved in Japan
Actemra was first deployed in Japan when Chugai (part of the Roche Group) filed for approval for its use as a treatment for rheumatoid arthritis in adult patients and for children with SJIA.
Filing was based on phase III data from trials conducted in Japanese patients that showed first-line use of Actemra significantly improved signs and symptoms of rheumatoid arthritis while reducing disease progression.
In April 2008, the company received the green light from the Japanese authorities to market the drug for rheumatoid arthritis.
Actemra was also approved in Japan for the treatment of Castleman’s disease, a rare condition that causes severe lymph node enlargement.
Approval in Japan hinted at success in other countries. Submissions were made to the FDA and the European Medicines Agency in November 2007. The filings were based on a substantial body of trial data which showed that Actemra significantly reduced signs and symptoms of rheumatoid arthritis when use either alone or in combination with standard treatments.
Worldwide Actemra approval status
Actemra is approved in the EU, Japan, Switzerland, India, Brazil, Kuwait, Peru, Moldova and Liechtenstein.
Phase III trials support clinical efficacy
Phase III trials were conducted in 4,000 RA patients in 41 countries including the US.
Data from phase III trials conducted in Japan have shown that Actemra is an effective therapy for rheumatoid arthritis and SJIA. In the pivotal Japanese trials in adult patients with rheumatoid arthritis, in which Actemra was compared with established disease modifying anti-rheumatic drugs, patients in the Actemra arm of the study achieved significantly greater improvement in signs and symptoms of rheumatoid arthritis as well as significantly less radiographic joint destruction (p=0.001).
Similar positive results were reported from pivotal phase III trials conducted outside Japan. These trials included OPTION (TOcilizumab Pivotal Trial in Methotrexate Inadequate responders), a three-arm, randomised, double-blind, controlled study designed to compare the safety and efficacy of Actemra plus methotrexate with methotrexate plus placebo in rheumatoid arthritis patients who had an inadequate response to methotrexate alone, and RADIATE (RheumAtoiD ArthritIs Study in Anti-TNF FailurEs). Both trials were successful and met their primary endpoints.
Phase III TOWARD (Tocilizumab in cOmbination With traditional DMARD therapy) trial was conducted to compare the safety and efficacy of Actemra in combination with Traditional Disease Modifying Drugs (DMARDs). The trial was conducted at 130 centres and recruited 1,216 patients with moderate to severe RA.
Results from the trial indicated that people treated with Actemra in combination with DMARDs experienced significant improvement when compared with those treated with DMARDs alone.
The phase III AMBITION (Actemra versus Methotrexate double-Blind Investigative Trial In mONotherapy) study was conducted in 673 patients to compare the effect of Actemra with methotrexate. Actemra showed higher efficacy to that of methotrexate in the trial.
The LITHE study was conducted in 15 countries. The randomised, double-blind, placebo-controlled study recruited 1,196 RA patients.
Results of the trial released in May 2008 indicated that the drug inhibited and slowed the structural damage of the joint.
The TENDER trial was conducted in 112 children suffering from SJIA at 43 sites in 17 countries. Children who were administered Actemra showed significant improvements compared to the placebo group.
The phase IIIb ACT-RAY study was conducted to compare the safety and efficacy of the Actemra monotherapy with the Actemra plus methotrexate therapy. Results indicated that the clinical efficacy of Actemra was comparable to that of Actemra in combination with methotrexate.
Phase IV studies will be initiated for a period of 32 weeks and will be completed by June 2014. More than 500 patients will be recruited for the studies, which will evaluate the safety and efficacy of the drug in RA patients.
Actemra was generally well tolerated by patients enrolled in the Japanese and international clinical trials programme.
Marketing commentary
Rheumatoid arthritis is a chronic and progressive autoimmune disease for which patients often require long-term therapy.
Biological response modifiers offer the prospect of not only providing symptom relief, but also the potential to stop disease progression, the ultimate goal of therapy.
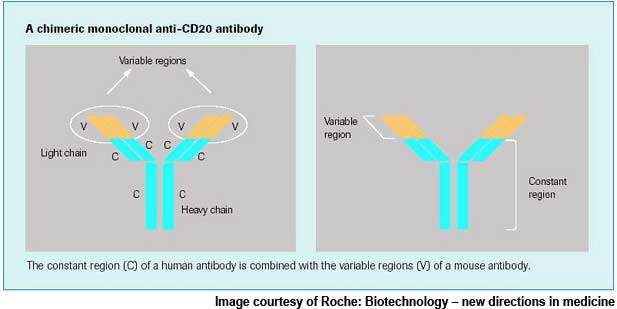
In addition to Actemra, Roche has also been exploring the potential of its targeted B-cell therapy MabThera / Rituxan (rituximab) as a treatment for rheumatoid arthritis. The therapy is currently marketed for the treatment of B-cell lymphomas.
In March 2006, Roche secured FDA approval for use of MabThera / Rituxan (rituximab) in adult patients with rheumatoid arthritis unresponsive to current biological therapies, which is one of the most difficult groups of rheumatoid arthritis patients to treat.
Roche in rheumatoid arthritis
Roche is working on a pipeline of potential drug candidates for the treatment of rheumatoid arthritis using several approaches. It is exploring MabThera, the selective B-cell therapy for rheumatoid arthritis, which provides a fundamentally different treatment approach targeting B-cells.
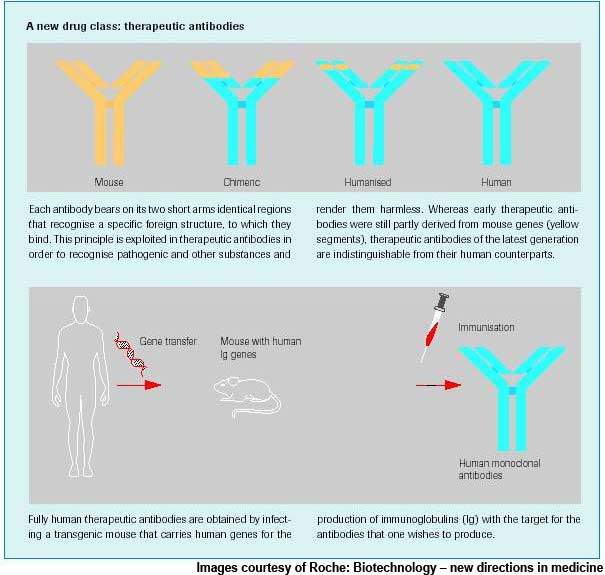
Actemra is a humanised monoclonal antibody to the IL-6 receptor, inhibiting the activity of IL-6, a protein that plays a key role in the rheumatoid arthritis inflammation process.
Roche had begun phase III development of ocrelizumab, a humanised anti-CD20 antibody, for rheumatoid arthritis.
The drug initially showed positive results in phase III studies in December 2009; however, in May 2010, Roche had to suspend development of the drug for this indication after adverse side effects and deaths were reported. Roche will continue with phase II studies of ocrelizumab for multiple sclerosis.
Pro-inflammatory cytokines as therapeutic targets
Biological response modifiers, which target inflammatory mediators of rheumatoid arthritis, represent a relatively new approach to the treatment of the condition and, indeed, of other autoimmune diseases.
Medications targeting tumour necrosis factor alpha (TNF-a), a pro-inflammatory cytokine implicated in the pathogenesis of rheumatoid arthritis, were among the first to be developed and approved for the treatment. Several anti-TNF-a medications are marketed for the treatment of rheumatoid arthritis.
Actemra differs from currently marketed biological response modifiers in targeting interleukin-6 (IL-6), a cytokine that is over-produced in the joints of rheumatoid arthritis patients.
Il-6 is believed to contribute to inflammation, swelling and joint damage and possibly thrombocytosis in rheumatoid arthritis. It is also elevated in the serum of patients with systemic onset juvenile idiopathic arthritis and believed to contribute to clinical signs and symptoms of this disease.
Systemic onset juvenile idiopathic arthritis affects about 10-20% of children with juvenile inflammatory arthritis.
Actemra is a humanised anti-human IL-6 receptor monoclonal antibody that works by competitively blocking the binding of IL-6 to its receptor. Thus, it inhibits the proliferative effects of IL-6, which leads to synovial thickening and pannus formation in rheumatoid arthritis.
Unmet clinical need in rheumatoid arthritis
Rheumatoid arthritis is a relatively common autoimmune disease, estimated to affect about 1% of the world’s population.
The disease is characterised by aberrant immune mechanisms that lead to joint inflammation and swelling with progressive destruction of joints.
In addition to affecting the joints, rheumatoid arthritis can also affect connective tissue in the skin and organs of the body.
Traditional approaches to the treatment of rheumatoid arthritis include the use of non-steroidal anti-inflammatory drugs, COX-2 inhibitors and disease-modifying anti-rheumatic drugs such as methotrexate.
However, none of these traditional approaches to treatment is ideal, especially in long-term use. Although treatment has advanced with the recent introduction of biological response modifiers, rheumatoid arthritis remains a disease for which there is still significant unmet clinical need.