Almorexant is the first member of a new class of drugs called orexin receptor antagonists indicated for the treatment of sleep disorders. It was discovered and developed by the Swiss pharmaceutical company Actelion.
In July 2008, Actelion signed a development and marketing agreement with GSK, whereby GSK secured exclusive worldwide rights to co-develop and co-commercialise almorexant for primary insomnia as well as to explore its potential in other orexin-related disorders.
In December 2009, Actelion announced positive results from its Phase III study RESTORA I. The study reached its primary end point, almorexant being effective in treating wake-after-sleep onset (WASO) in comparison with placebo.
However, on 28 January 2011, GSK and Actelion discontinued the clinical development and long-term Phase III studies of almorexant due to the occurrence of side effects.
Orexins in the sleep-wake cycle
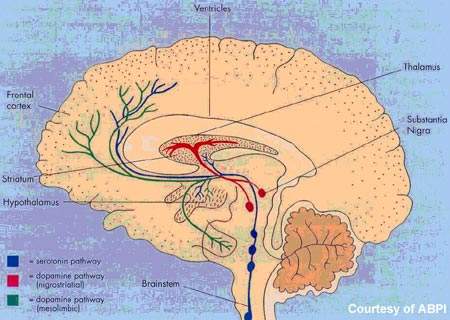
Orexins, or hypocretins as they were previously known, are neuropeptides produced by the hypothalamus. In addition to the regulation of appetite, orexins are now known to maintain wakefulness and thus play a pivotal role in controlling the sleep-wake cycle.
There is also evidence to suggest that narcolepsy, a debilitating sleep disorder, is associated with deficiencies in orexinergic function. These discoveries opened the door to the possibility of producing orexin-related medications for the treatment of sleep disorders.
Almorexant is an oral orexin receptor antagonist. It penetrates the blood-brain barrier, where it induces transient and reversible blockade of the orexin receptors, OX1 and OX2.
In a proof-of-concept study in 161 patients with primary insomnia, almorexant 100–400mg produced significant, dose-dependent, improvements in sleep efficiency (primary endpoint) as well as improvements in latency to persistent sleep (LPS) and wake-after-sleep onset (WASO), for which the study was not powered. Consistent with previous safety and tolerability studies in healthy volunteers, almorexant was well tolerated by patients in the proof-of-concept study.
Towards better drugs for insomnia
Estimated to affect between 20% and 30% of the general population, sleep disorders are extremely common. Treatments range from self-medication with non-pharmaceutical preparations to prescription medications, such as benzodiazepines, antidepressants, antihistamines and most recently selective hypnotics. Although the new generation of selective or dedicated hypnotic agents represent a major advance in the treatment of insomnia, they still carry some risk of dependence.
Ideally, drug therapy for insomnia should induce sleep that is qualitatively close to normal sleep cycles and be devoid of rebound and withdrawal effects.
Clinical studies suggested that targeting the orexin system may produce beneficial treatment effects, helping patients with insomnia to both fall and stay asleep. Importantly, it appears that increased sleep efficiency is achieved without adverse effects on next-day performance, an acknowledged drawback to currently available drugs that target benzodiazepine receptors.
Phase III development discontinued
Following the successful proof-of-concept study in primary insomnia, almorexant underwent extensive evaluation in the RESTORA (REstore normal physiological Sleep with The Orexin Receptor antagonist Almorexant) registration programme.
The first trial, RESTORA 1, for which Actelion announced positive results, evaluated the efficacy and safety of almorexant in adults with chronic primary insomnia. This international, multicentre, randomised, placebo-controlled trial also included an active-reference arm in which patients were randomised to zolpidem, a GABA-A receptor modulator.
Primary objectives were to compare the effects of almorexant with placebo on LPS and WASO. Additional Phase III trials involving almorexant focused on longer treatment exposure, additional exploratory endpoints, and special populations such as elderly patients (≥65 years) in whom sleep disorders were common. A review of data from the additional studies, however, prompted the company to discontinue the clinical development of the drug.
Marketing commentary
In the US alone it is estimated that more than 40 million people suffer from chronic sleep disorders, while a further 20 million suffer occasional sleep problems. For years conventional benzodiazepines were the mainstay of pharmacological treatment of insomnia. Gradually they have been replaced by the new selective hypnotics, which now dominate the market for drugs to treat sleep disorders and continue to drive its growth.
However, good opportunities exist for new drugs to treat sleep disorders, especially where they offer benefits over existing agents.