Alofisel® (formerly Cx601 / darvadstrocel) is the first allogeneic stem cell therapy to be approved for the treatment of complex perianal fistulas in adult patients with Crohn’s disease.
The drug was discovered by TiGenix NV and co-developed by Takeda Pharmaceutical Company.
Alofisel received positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) and the Committee for Advanced Therapies (CAT) in December 2017.
The drug was granted orphan designation by the European Commission (EC) and the US Food and Drug Administration (FDA) in 2009 and 2017 respectively.
It subsequently received marketing authorisation (MA) in Europe from the EC in March 2018.
The FDA approval for Alofisel is currently under review.
Perianal fistulas in Crohn’s Disease
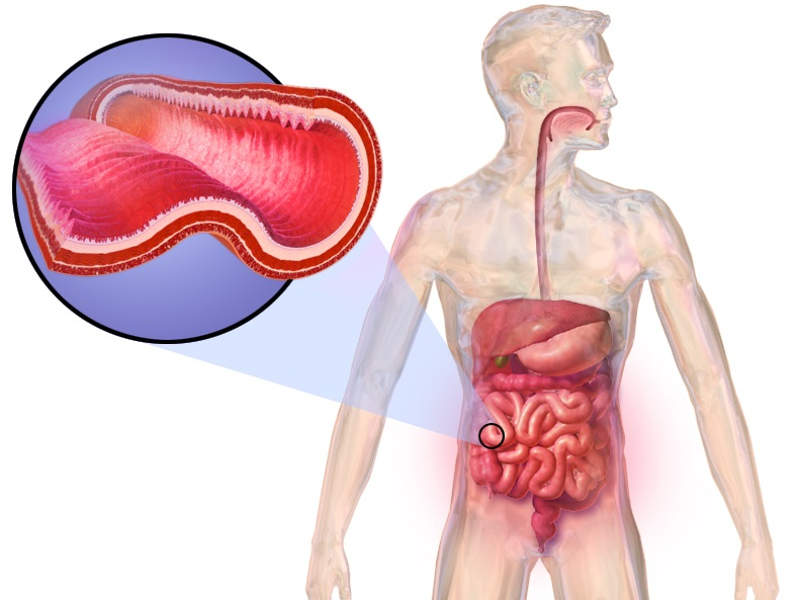
Crohn’s disease is a prolonged inflammatory disease of the intestine.
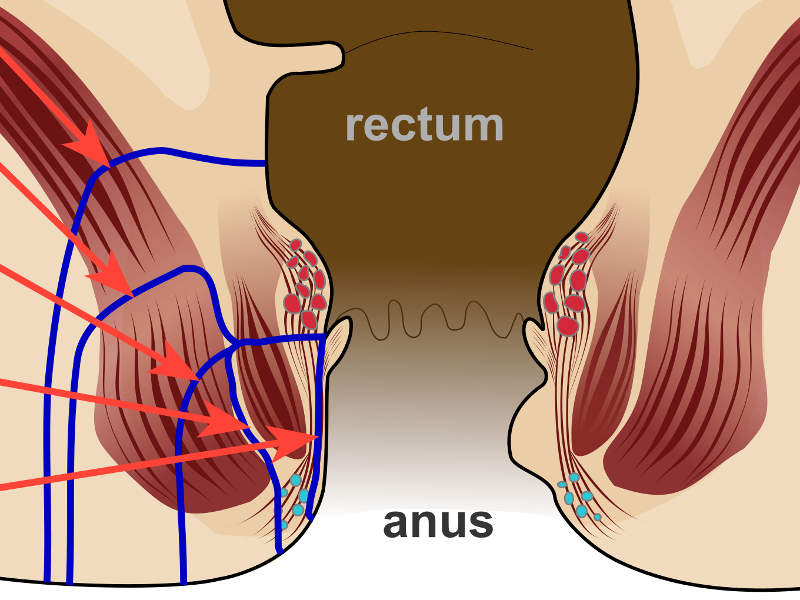
The most common complication found in patients suffering from Crohn’s disease, Perianal fistulas, is an abnormal connection between the perianal space and the outside skin surface.
The people suffering from the disease tend to be affected by perianal fistulas due to the inflammation that pierces into the whole thickness of the bowel wall.
Crohn’s disease is considered to be complex when its treatment involves a high risk of causing anal incontinence.
Perianal fistulas typically occur in Crohn’s disease patients between the ages of 20 and 40.
Alofisel’s mechanism of action
Alofisel contains allogeneic (donor-derived) expanded adipose-derived stem cells (eASCs), which show immunomodulatory and anti-inflammatory effects at inflammation sites.
The drug impairs proliferation of activated lymphocytes, while its immunoregulatory properties reduce the inflammatory cytokines.
It is currently available in the form of a suspension for injection.
Clinical trials on Alofisel
The EC’s approval for Alofisel was based on results obtained from a pivotal phase-three clinical trial named ADMIRE-CD.
The study was a randomised, double-blind, parallel-group, placebo-controlled, multi-centre clinical trial.
A total of 212 patients took part in the clinical study, including 205 that were administered a local intralesional injection of either Alofisel 120 million cells or placebo in a 1:1 ratio.
The trial was completed in August 2015.
The results of the study demonstrated that patients treated with Alofisel met the primary endpoint of combined remission at week 24.
Secondary endpoints included clinical remission and response at week 24.
Patients who received Alofisel showed a 44% greater probability of achieving combined remission compared to those given the placebo.
Further follow-up data showed that Alofisel maintained the long-term remission of treatment-refractory complex perianal fistulas in patients with Crohn’s disease over 52 weeks.
A global phase-three clinical trial on Alofisel, named ADMIRE-CD II, was initiated in 2017 in support of the US Biologic License Application (BLA) submitted to the FDA.
The trial is a randomised, double-blind, placebo-controlled study aimed at evaluating the efficacy and safety of a single administration of Alofisel for the treatment of complex perianal fistulas in Crohn’s disease patients.
Marketing commentary on TiGenix
TiGenix NV is an advanced biopharmaceutical company headquartered in Leuven, Belgium, whose lead product is Alofisel.
The organisation is primarily engaged in the development of novel therapies for serious medical conditions through the anti-inflammatory properties of allogeneic, or donor-derived, stem cells.
TiGenix granted Takeda the rights to develop and commercialise Alofisel outside the US in July 2016.
TiGenix is set to receive a milestone-based payment of €15m ($18.5m) from Takeda following the marketing authorisation of the drug.
Other medications available in the market for the same indication include Stelara (Ustekinumab) developed by Janssen Biotech and Entyvio (vedolizumab) manufactured by Takeda Pharmaceuticals.