Under development by Amira Pharmaceuticals, AM103 is a novel inhibitor of 5-lipoxygenase activating protein (FLAP) that is being investigated for the treatment of respiratory disorders such as asthma and allergic rhinitis.
In January 2010, Amira announced the development of a solid dose formulation, a significant landmark in the FLAP development programme. The formulation is vital for future development and marketing of the company’s FLAP inhibitors including AM103.
Amira Pharmaceuticals has entered into an agreement with GlaxoSmithKline (GSK) in January 2008 to latter to produce, commercialise and develop FLAP inhibitors for the treatment of inflammatory diseases.
AM103 is undergoing Phase II clinical trials for treating respiratory disorders under the supervision of GSK.
On 13 July 2010, AM103 was grated patents by the UK Intellectual Property Office.
Targeting the leukotriene pathway
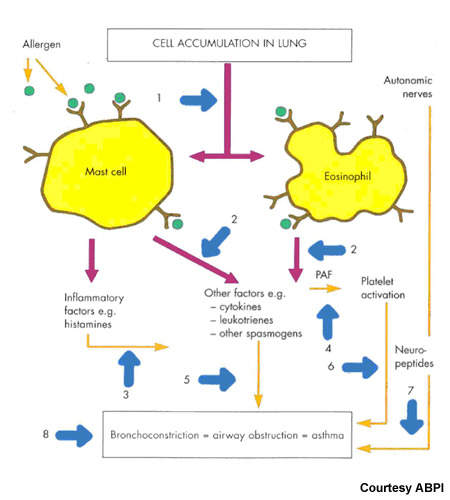
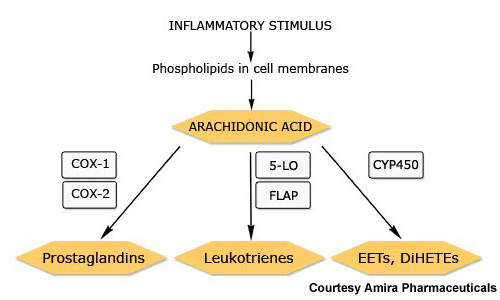
Together with prostaglandins and other arachidonic acid-derived lipids, leukotrienes are constituents of the eicosanoid family of inflammatory mediators. They play an important role in inflammatory diseases, such as asthma.
Blocking the pro-inflammatory effects of leukotrienes is a logical therapeutic strategy for treatment of asthma and other inflammatory-related disorders.
In fact, leukotriene receptor antagonists (montelukast, pranlukast and zafirlikast) are already approved medications for the treatment of asthma and of concomitant allergic rhinitis in asthma patients.
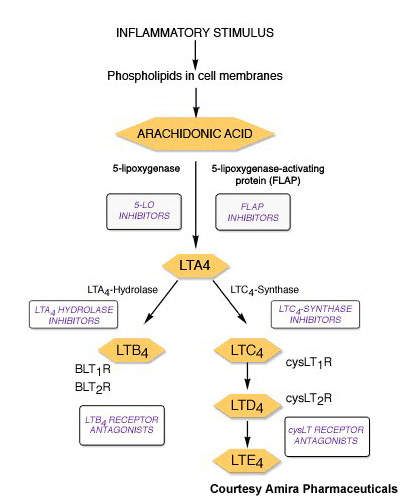
Currently available leukotriene receptor antagonists act via the type 1 leukotriene (CysLT1) receptor. By contrast, FLAP inhibitors target an earlier stage in the leukotriene pathway and therefore have potential to inhibit production of a broader spectrum of leukotrienes with perhaps the offer of greater efficacy.
AM103 works by binding to FLAP and thereby inhibiting the synthesis of proinflammatory leukotrienes.
AM103 enters clinical trials
With the completion of a Phase I safety trial in healthy volunteers, AM103 has emerged as a safe and well-tolerated drug at doses in the range 50mg to 1,000mg/day. The double-blind, placebo-controlled Phase I trial also investigated the pharmacodynamic properties of AM103 and most specifically its affect on leukotriene (LT) production. Over the 11-day study, systemic exposure to AM103 increased linearly across the dose range accompanied by dose-dependent reductions in LTB4 and LTE4.
Based on the success of this initial clinical trial the company is carrying out Phase II trials with AM103 in asthma patients. Given the drug’s long half-life of up to ten hours, there is scope to evaluate once-daily oral dosing. Clinical trials of similar design are also underway with AM803, Amira’s second oral FLAP inhibitor.
Amira forges partnership with GSK
In early 2008, GSK secured an exclusive worldwide license to the further development, manufacture and commercialisation of Amira’s FLAP inhibitors, with the initial focus on AM103 – its most advanced drug.
With the backing of GSK, Amira hope that AM103 will become the first FLAP inhibitor to market as a new treatment for asthma patients. This seems feasible given that leukotriene receptor antagonists are already established treatments for asthma and concomitant seasonal rhinitis. However, it will be interesting to see if this new class of drugs offers efficacy or safety benefits over the selective CysLT1 receptor antagonists.
GSK is already a major player in the respiratory market with Advair, its market leading combination corticosteroid and long-acting beta antagonist. The company is therefore well-placed to exploit the potential of AM103.
Marketing commentary
Despite the success of inhaled steroids and beta-agonists in the treatment of asthma, there remains a need for alternative, non-steroidal, therapies to treat a disease that affects more than 300 million people.
Targeting the leukotriene pathway has already proved successful with the advent of oral leukotriene receptor antagonists, the sales of which have approached those of inhaled corticosteroids in some markets. FLAP inhibitors, drugs that interfere directly with leukotriene production, could also prove successful especially if they provide greater efficacy to existing leukotriene receptor antagonists.