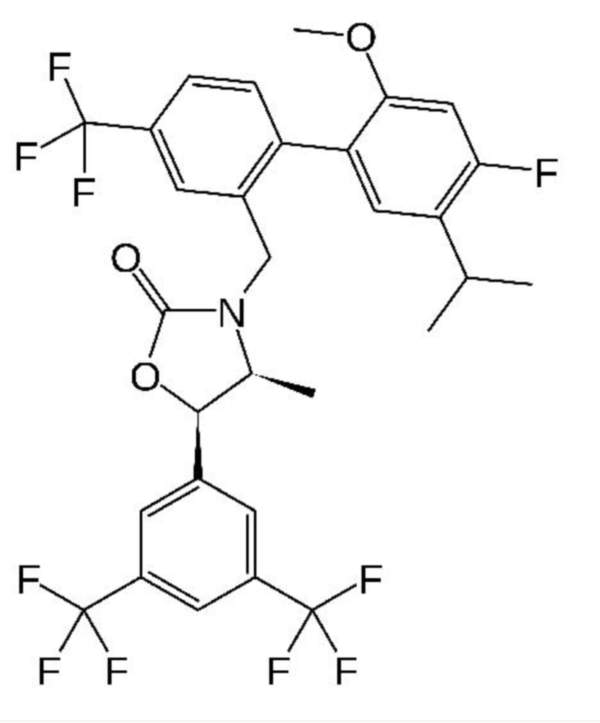
Anacetrapib (MK-0859) is an orally active cholesteryl ester transfer protein (CETP) inhibitor indicated for the treatment of atherosclerosis.
It is also targeted at related diseases such as coronary heart disease (CHD) and dyslipidemia. It is being developed by Merck and is currently in Phase III development status.
Atherosclerosis, its causes and its effects
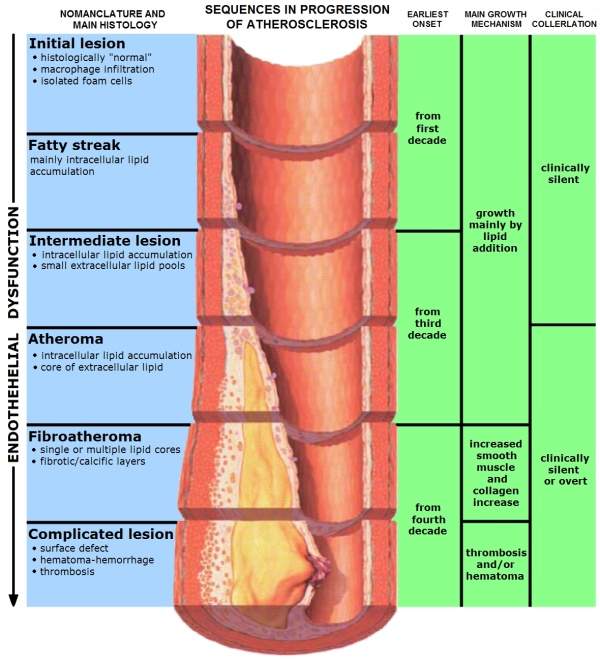
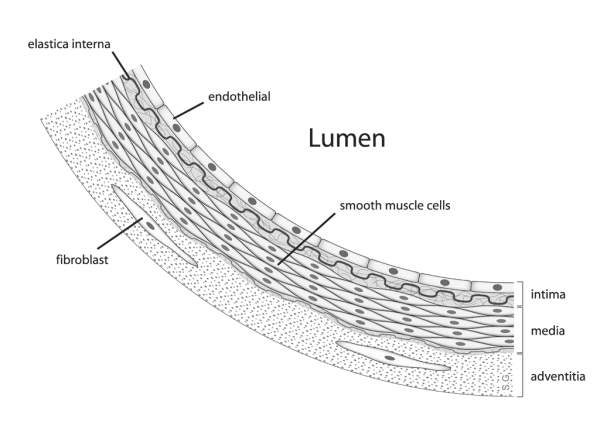
Atherosclerosis is a condition wherein the arteries of the heart harden due to the formation of structures called plaques. These structures are formed due to the build up of fat, cholesterol and other matter in the arteries.
As the plaques start to build in the artery, they ultimately block them, leading to several heart problems including CHD. In some cases, the plaques can rupture and move across the body leading to several complications.
When they move into the lungs they can cause pulmonary embolism. They can also cause heart attacks by blocking the arteries of the heart or cause strokes by blocking the arteries of the brain.
Atherosclerosis is mainly caused by an unhealthy lifestyle – eating food high in cholesterol, intake of alcohol, lack of exercise and being overweight. In such individuals, a condition called dyslipidemia is prevalent which can lead to atherosclerosis.
In individuals diagnosed with dyslipidemia, elevated levels of low-density lipoprotein cholesterol (LDL-C) and/or triglycerides or low high-density lipoprotein cholesterol (HDL-C) levels are observed.
Plaques, once they are formed, cannot be reversed but their formation can be slowed by making changes in one’s lifestyle, by exercising and having a healthy diet.
Anacetrapib – cholesteryl ester transfer protein inhibitor
CETP inhibitors such as anacetrapib are plasma proteins which help in the exchange of cholesteryl esters and triglycerides between HDL and lipoproteins.
Anacetrapib works by lowering LDL by nearly half while boosting HDL at the same time. It does not cause any side effects such as increasing blood pressure which was caused by torcetrapib, another drug of the same class from Pfizer.
Clinical trials of Merck’s atherosclerosis treatment anacetrapib
Anacetrapib has showed its efficacy in consistently increasing HDL-C concentrations and was also well tolerated in preclinical trials.
Five Phase I trials have been conducted on anacetrapib to test the safety, tolerability and pharmacokinetics in patients suffering from dyslipidemia who are on statin therapy, hepatic insufficiency, impaired renal function and primary or mixed hyperlipidemia.
Phase II studies of anacetrapib included a dose ranging study in patients with primary hypercholesterolemia or mixed dyslipidemia. The study recruited 500 patients and was conducted between June 2006 and February 2007.
Another Phase II study evaluated the effect of anacetrapib as a monotherapy and in combination with atorvastatin in Japanese patients with dyslipidemia. The study commenced in September 2009 and was completed in May 2010. It enrolled 400 patients.
Two Phase III studies of anacetrapib are currently ongoing. The DEFINE (Determining the EFficacy and Tolerability of CETP INhibition with AnacEtrapib) study is evaluating the safety, tolerability and efficacy of anacetrapib in patients with CHD or CHD equivalent disease. It will assess the lipid-modifying capability of anacetrapib along with ongoing statin therapy or other lipid-modifying agents.
About 1,623 patients have been recruited from 20 countries for the trial which commenced in April 2008. The study is expected to be completed by February 2013.
Positive results announced from the DEFINE study in November 2010 indicated that anacetrapib was able to reduce LDL by 40% and increase HDL by 138%. Cardiovascular events were not elevated in the study.
The second Phase III trial of anacetrapib is the REVEAL (Randomised EValuation of the Effects of Anacetrapib Through Lipid-modification) study. The study will provide insights into whether the changes in cholesterol levels will translate into clinical benefit for patients. It started in June 2011 and will enrol 30,000 patients. It is expected to be completed by January 2017 with initial results expected in 2015.
Marketing commentary for anacetrapib and CETP inhibitors in general
Pfizer’s torcetrapib was the first in class of CETP inhibitors but its development was halted after safety concerns were raised in Phase III studies. The failure of torcetrapib raised questions about the safety of other CETP inhibitors. With anacetrapib demonstrating no major safety concerns, the future of the drug looks promising.