Andexxa® (coagulation factor Xa recombinant, inactivated-zhzo) is an intravenous infusion indicated for the treatment of patients with factor Xa inhibitor-associated acute major bleeding.
The drug was discovered and developed by Portola Pharmaceuticals, a biopharmaceutical company based in the US.
A biologics license application (BLA) for Andexxa® was accepted for review by the US Food and Drug Administration (FDA) in February 2016. The FDA approved the drug in May 2018.
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) is expected to give a formal opinion on Andexxa® by the end of 2018 and the European Commission (EC) is expected to issue its decision in early-2019.
Andexxa’s mechanism of action
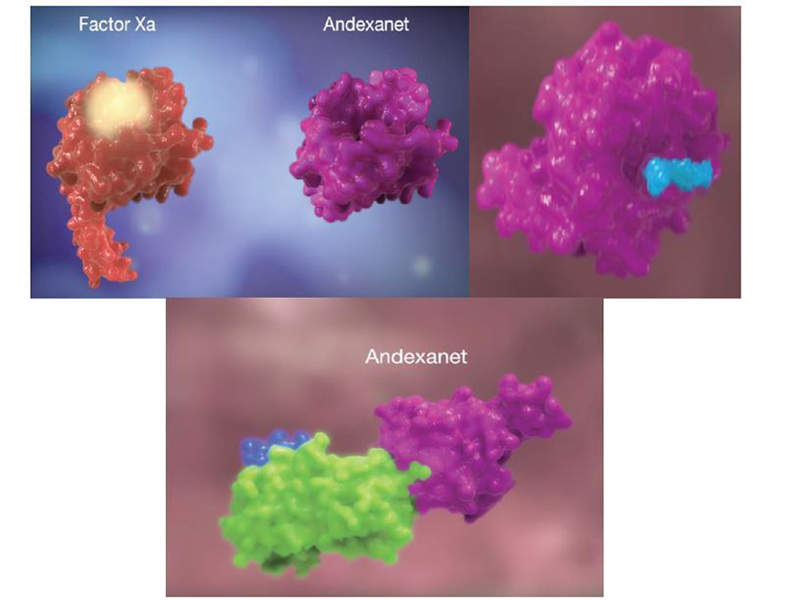
The andexanet alfa contained in Andexxa® is a modified recombinant protein that blocks factor Xa inhibitors rivaroxaban and apixaban, and reverses their anticoagulant effect.
The Andexxa protein also has the ability to bind and inhibit the activity of tissue factor pathway inhibitor (TFPI), which increases tissue factor-initiated generation of thrombin.
The drug is available as a 100mg lyophilised powder in a single-use vial for intravenous injection.
Clinical trials on Andexxa
The FDA’s approval of Andexxa® is supported by data from two Phase III studies named ANNEXA-A and ANNEXA-R, which revealed the successful reversal of anticoagulant activity for factor Xa inhibitor anticoagulants Xarelto (rivaroxaban) and Eliquis (apixaban).
Also known as the apixaban reversal study, ANNEXA-A enrolled patients with a median age of 57 years. The patients were administered with 5mg of apixaban twice daily for 3.5 days. Three hours after the last apixaban dose, eight patients were randomised to placebo and 24 were administered with Andexxa® as a 400mg intravenous injection followed by a 4mg per minute infusion for two hours.
Also known as rivaroxaban reversal study, the ANNEXA-R study enrolled patients with a median age of 57 years, which were administered with rivaroxaban 20mg once daily for four days. Four hours after the last rivaroxaban dose, 13 patients were randomised to placebo, while 26 received Andexxa® as an 800mg intravenous bolus injection followed by an 8mg per minute infusion for two hours.
The primary endpoint of both the studies was the percentage change in anti-FXa activity from baseline, which was a median decrease in anti-FXa activity from baseline of 97% for the rivaroxaban reversal study and 92% for the apixaban reversal study.
Portola is also carrying out the ANNEXA-4 trial, which is designed as a multinational, single-arm, open-label, multi-centre cohort study intended to test the safety and efficacy of Andexxa® in patients with FXa inhibitor-associated major bleeding.
The most common adverse reactions associated with the use of Andexxa® were urinary tract infections and pneumonia. Infusion-related reactions were also reported in some patients during the trial.
Anticoagulants and their side effects
Also known as blood thinners, anticoagulants help prevent blood clots and lower the risk of heart stroke and pulmonary embolism.
Traditional anticoagulants prevent blood clots by reducing specific proteins called clotting factors produced in the liver using vitamin K. Anticoagulants work by interfering with vitamin K and blocking the production of specific clotting factors thereby preventing the formation of clots.
Recently developed anticoagulants such as apixaban, dabigatran, edoxaban and rivaroxaban work differently when compared to traditional anticoagulants. They interfere with the production of thrombin and Factor Xa, which play a major role in blood clotting.
Use of anticoagulants can lead to severe bleeding in some cases. Potential risks associated with anticoagulants include bleeding in various parts of the body leading to complications such as rash, bloating, diarrhoea and jaundice.
Marketing commentary on Portola Pharmaceuticals
Headquartered in South San Francisco, California, Portola Pharmaceuticals is focused on the discovery, development and commercialisation of therapies that transform patient lives. It provides advance patient care in thrombosis and other hematologic diseases.
The company owns commercial-stage assets Bevyxxa® (betrixaban) and Andexxa®.
Bevyxxa (betrixaban) is an oral, once-daily factor Xa inhibitor that was approved by the FDA in mid-2017. It is the company’s first non-vitamin K antagonist oral anticoagulant (NOAC) drug.
Portola is currently developing Cerdulatinib, an investigational SYK/JAK inhibitor, for the treatment of hematologic cancers.