Developed by Arena Pharmaceuticals, APD125 is an oral serotonin antagonist with potential for the treatment of insomnia. In December 2008, Arena announced that APD125 did not meet its primary or secondary endpoints in the Phase IIb trial initiated in April 2008. The treatment with APD125, however, was well tolerated among the patients and no untoward events or emerging safety results were reported as compared to placebo.
Arena discontinued the development of APD125 following the results of the Phase IIb trial.
New targets for sleep disorders
For years conventional benzodiazepines were the mainstay of pharmacological treatment of insomnia. Selective gamma-amino butyric acid (GABA)A receptor modulating drugs, or non-benzodiazepine hypnotics, which now dominate the market for insomnia have largely replaced the older benzodiazepines.
Although non-benzodiazepine hypnotics address some of the drawbacks inherent in the benzodiazepines, which also target the GABA receptor complex, they are not without side effects. For example, they can cause adverse effects such as ‘hangovers’, rebound insomnia and, more rarely, short-term memory loss. Moreover, in the US, GABA-A receptor drugs are DEA-scheduled, controlled substances because of their potential for abuse and dependency.
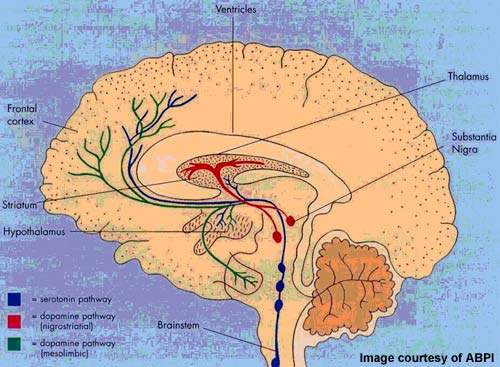
Drawbacks associated with current drugs have stimulated the search for different therapeutic targets to treat insomnia and other sleep disorders. Considerable research interest has focused on 5-HT receptors as a possible therapeutic target and several serotonin antagonists and agonists are in development for insomnia.
Arena’s APD125 was a potent serotonin antagonist that selectively targeted the 5-HT2A receptor. Available research suggests that serotonin receptor antagonism may be effective in maintaining sleep but without the concomitant ‘hangover effect’, of next day drowsiness that is often seen with current hypnotics.
Efficacy demonstrated in early trials
Results from the Phase IIa trial, in which APD125 was administered at night time to patients with chronic insomnia, showed significant improvements in a number of efficacy endpoints including Wake After Sleep Onset, Wake Time During Sleep, and number of awakenings and arousals.
APD125 also significantly increased the time spent in the deep sleep phase of the sleep cycle and decreased the amount of time spent in light sleep. APD125 was well tolerated by patients in this study with no evidence of next day cognitive impairment.
The Phase IIb trial evaluated the efficacy and tolerability of the drug in patients suffering from primary insomnia. The trial enrolled 675 primary insomnia patients in about 70 clinical centres in the US. The primary endpoint of the trial was change from baseline in number of awakenings after sleep onset (sNAASO).
Large market to tap
Estimated to affect between 20% and 30% of the general population, sleep disorders are extremely common. Not surprisingly drugs for the treatment of insomnia, the most common sleep disorder, constitute an already large and growing market. Current estimates suggest the market is worth over $6bn a year.
Today treatments for insomnia range from self-medication with non-pharmaceutical preparations to prescription medications, such as benzodiazepines, antidepressants, antihistamines and the more recent selective hypnotics, such as zaleplon, zolpidem and zopiclone (the so-called Z drugs).
The anticipated arrival of a new generation of drugs targeting serotonin receptors is expected to be a potent stimulus to further market growth and development, especially if they address deficiencies in current medications for sleep disorders.
Marketing commentary
Sleep disorders remain poorly understood, under-diagnosed and under-treated. Good opportunities exist for new drugs to treat sleep disorders, especially where they offer benefits over existing agents. Ideally, drug therapy for insomnia should induce sleep that is qualitatively close to normal sleep cycles and be devoid of rebound and withdrawal effects.
If the new generation of serotonin antagonists/agonists currently in development meet these needs, then they will seriously challenge the dominance of the selective hypnotics.