ASP-1929 is a first-in-class therapy indicated for the treatment of head and neck squamous cell carcinomas (HNSCC).
The drug is being developed by Rakuten Aspyrian, a clinical-stage biotechnology company headquartered in the US. ASP-1929 received Fast Track designation from the US Food and Drug Administration (FDA) for the treatment of HNSCC in the first quarter of 2018.
ASP-1929 is also being evaluated in different types of solid tumour, including oesophageal, lung, colorectal and pancreatic cancers.
Head and neck squamous cell carcinomas causes and symptoms
Squamous cell carcinoma is a type of cancer that affects the squamous cells, which occur in the outer layer of skin and the mucous membrane. HNSCC usually occurs in the mucous membranes of cells in the mouth, nose and throat.
HNSCC is caused by either smoking or chewing tobacco and heavy alcohol consumption. The symptoms of the disease include abnormal patches or ulcers in the mouth and throat, pain in the mouth, sinus congestion, a hoarse voice, or enlarged lymph nodes.
The disease can metastasize and spread to other parts of the body such as the lymph nodes or the lungs and can be fatal. HNSCC is the sixth most commonly diagnosed cancer type and the eighth most common cause of cancer death globally.
ASP-1929 mechanism of action
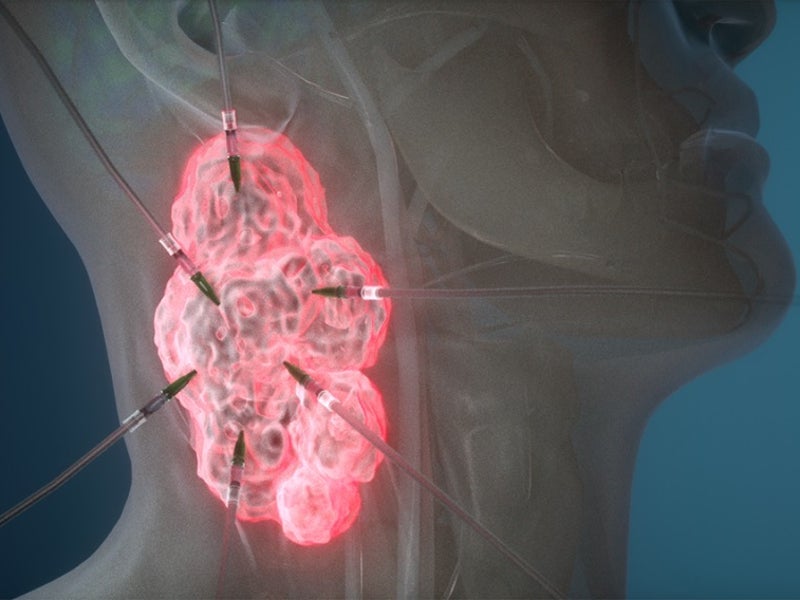
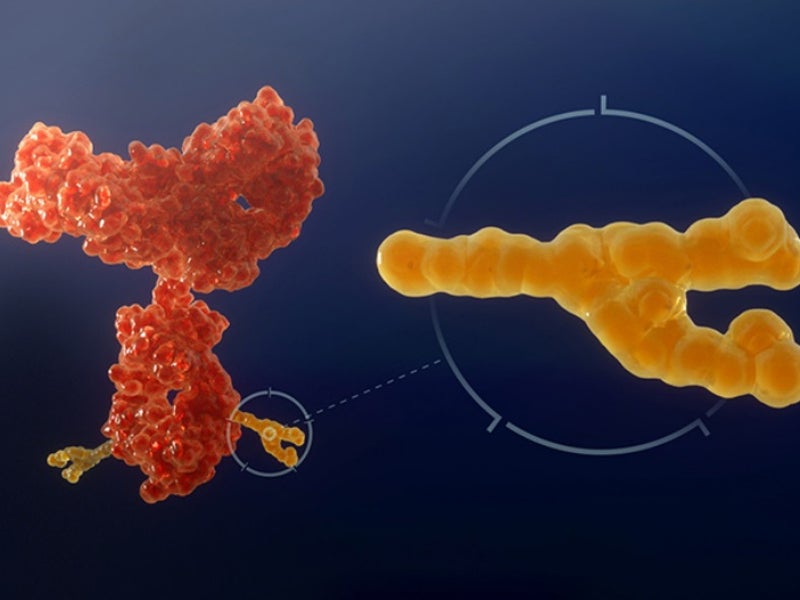
ASP-1929 is an antibody drug conjugate of cetuximab and IRDye 700DX®, a phthalocyanine dye. It targets epidermal growth factor receptor (EGFR), which is a known cancer antigen expressed in multiple kinds of solid tumours.
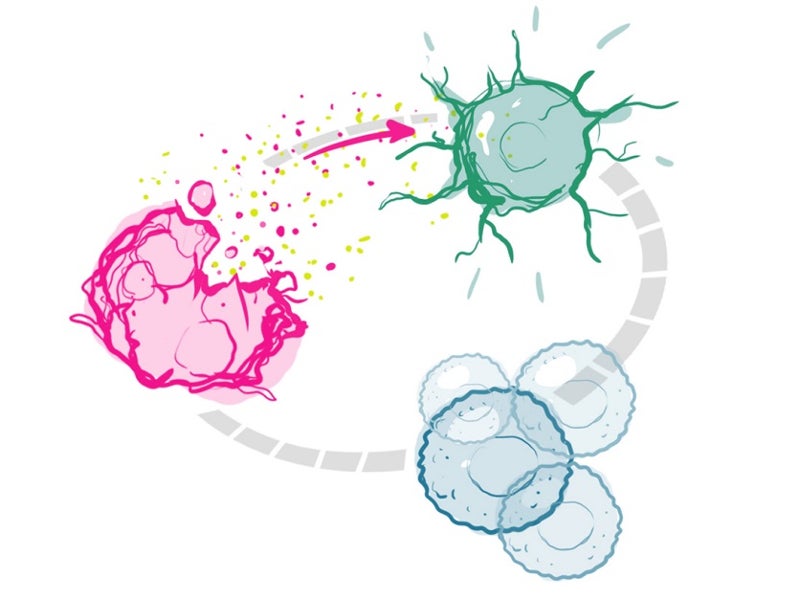
The drug binds to the tumour and is activated using a red light through a patented investigational laser and fibre-optics. The activation of the drug locally ensures that it targets the tumour, but not the normal tissues and structures.
Clinical trials on ASP-1929
A Phase I/ Phase II trial on ASP-1929 enrolled 24 patients with HNSCC to evaluate the safety and efficacy of the drug.
Iinitial results from the study demonstrated significant improvement in the objective response rate and improvements in progression-free and overall survival compared with the available historical data of existing treatments. Full results from the trial are expected in Q1 2019.
Enrolment for a Phase III clinical trial named LUZERA-301 is currently ongoing to test the safety and efficacy of the drug in patients with recurrent local HNSCC, who have failed two previous lines of therapy.
The trial is expected to enrol 275 patients across 75 centres in the US, Europe and Asia. Patients will be randomised to receive either ASP-1929 or investigator’s choice of systemic therapy in a 2:1 ratio.
The primary endpoints of the study include progression-free survival and overall survival, while secondary endpoints include objective response rate and complete response rate.
Additional Phase II trials to evaluate the safety and efficacy of the drug in other types of cancers are also planned to be undertaken.
Marketing commentary on Rakuten Aspyrian
Established in 2013, Rakuten Aspyrian is focused on the development of cancer therapies based on a new photo-immunotherapy platform, which was exclusively licensed from the National Institutes of Health.
ASP-1929 is the company’s lead drug candidate, which is being evaluated in various types of cancers. Other product candidates of the company include Antibody B-IR700 and Antibody C-IR700.