Aveed (testosterone undecanoate) is a testosterone replacement therapy indicated for the treatment of hypogonadism in adult men who are associated with a deficiency or absence of the male hormone testosterone.
The testosterone undecanoate was originally discovered by Bayer Pharma, which sold the licence for the development to Endo Pharmaceuticals in the US.
Endo received approval for Aveed from the US Food and Drug Administration (FDA) for treatment of hypogonadism in adult men in March 2014.
Targeting hypogonadism
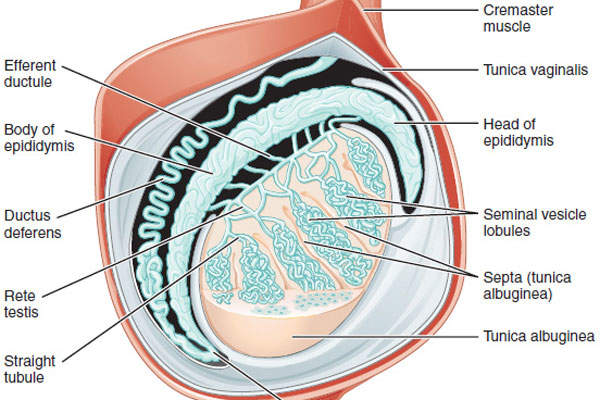
Hypogonadism is a condition that involves the body’s sex glands producing fewer or no hormones due to the reduced functional activity of the gonads. The disease, depending on the degree of severity, can result in partial or complete infertility.
How Aveed works in boosting testosterone
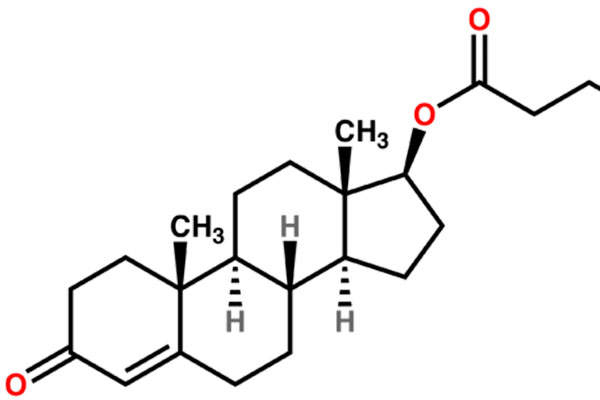
Aveed contains testosterone undecanoate, which is an ester of testosterone. The drug can be administered using androgen replacement therapy (ART). The testosterone hormone is primarily responsible for the normal growth and development of the male sex organs and for the maintenance of secondary sex characteristics.
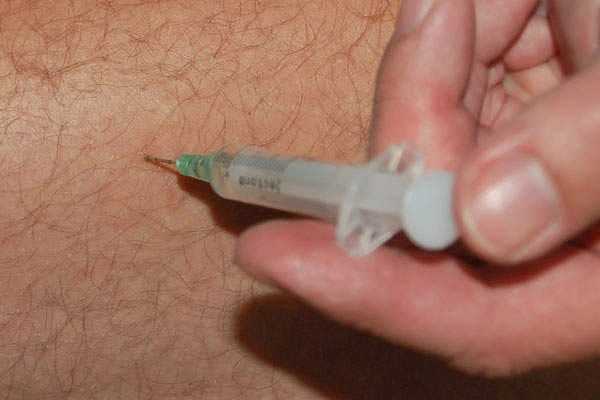
The drug is available in a 3ml (750mg) single-use vial, which can be administered through intramuscular injection.
Details of Aveed clinical trials
The FDA approval for Aveed was based on the results obtained from a Phase III clinical study conducted for 84 weeks. The single-arm, open label, multicentre clinical study enrolled 130 hypogonadal adult patients who had an average age of 54 years with morning serum total testosterone concentrations <300 nanograms per decilitre (ng/dL). The patients were administered with Aveed 750mg injection at the initiation of the therapy, at four weeks, and then every ten weeks thereafter.
The primary endpoint of the study was the percentage of subjects with average serum total testosterone concentration (Cavg) after the third Aveed injection. The secondary endpoint included the percentage of subjects with maximum total testosterone concentration (Cmax) above three predetermined limits greater than 1,500ng/dL.
The results of the study demonstrated that patients treated with Aveed showed increased mean serum testosterone levels and maintained them up to ten weeks at steady state. The study also found that about 94% of the patients administered with Aveed maintained a Cavg within the normal range between 300ng/dL and 1,000ng/dL. 117 patients out of 130 completed the study procedures through Week 24 after the third Aveed injection and were included in the evaluation of testosterone pharmacokinetics.
The adverse events found during the clinical study were acne, pain at the injection site, rise in prostatic specific antigen (PSA), rise in estradiol, hypogonadism, fatigue, irritability, increase in haemoglobin, insomnia, and mood swings.
The safety and efficacy of the testosterone undecanoate injection was evaluated through 18 clinical trials, which were conducted across the world by enrolling over 3,556 subjects outside the US.
Aveed marketing
Endo Pharmaceuticals, headquartered at Malvern in Pennsylvania, US, is an operating wing of Endo International. The company is engaged in the development, production and delivery of high-value branded pharmaceutical products that meet the unmet needs of patients.
Endo Pharmaceuticals holds the marketing rights of Aveed in the US and plans to launch the drug in the US market in March 2014. The drug is owned and marketed with trade name Nebido by Bayer Pharma across the world excluding the US. The drug has been approved in more than 86 countries.