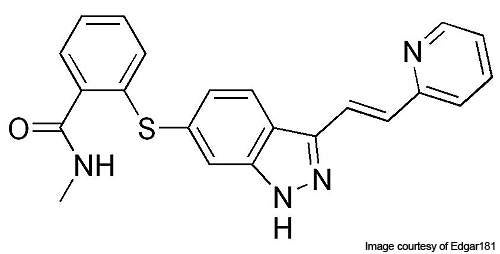
Inlyta (axitinib) was developed by Pfizer for treating advanced renal cell carcinoma (ARCC) and hepatocellular carcinoma. The drug was investigated as a single dose therapy, as well as in combination with chemotherapy.
Pfizer submitted a new drug application for the treatment of advanced renal cell carcinoma based on the Phase III AXIS 1032 trial.
The drug was accepted for review by the FDA in June 2011. Pfizer received approval for Inlyta from the FDA in January 2012 for the treatment of ARCC.
The drug was also accepted for review by the European Medicines Agency in June 2011. The review was based on a Phase III trial named AXIS 1032. Pfizer received marketing authorisation approval for Inlyta from the European Medical Authority (EMA) in May 2012 for the treatment of patients suffering from ARCC.
The drug also received marketing approvals for the treatment of ARCC in Australia and UK in 2012.It is also approved in Switzerland, Japan, Canada, and Korea.
Pfizer entered into an agreement with SFJ Pharma for the co-development of Inlyta (axitinib) in Asia.
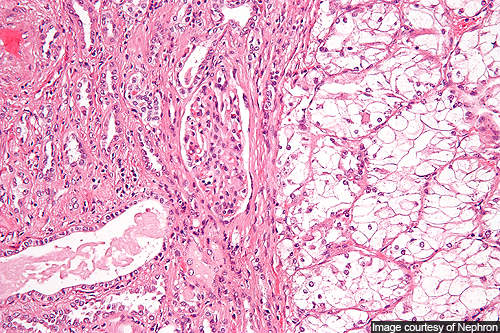
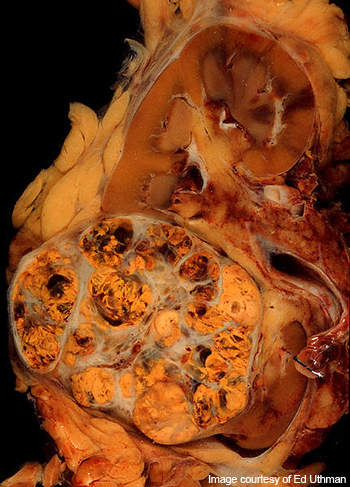
Renal cell carcinoma
Renal cell carcinoma is the cancer occurring in the lining of small tubules of the kidney. It is the most common type of kidney cancer in adults.
Though the exact cause of the cancer remains unknown, several risk factors like dialysis, family history of the disease, horseshoe kidney, high blood pressure, polycystic kidneys and smoking can cause it.
The symptoms of the disease include abdominal swelling and pain, swelling of the veins of testicles, back pain, weight loss and blood in urine. Pale skin, constipation, vision problems and cold intolerance may also be observed.
More than 210,000 people are diagnosed with renal cell carcinoma every year and 102,000 people die as a result of the cancer.
Axitinib’s mechanism of action
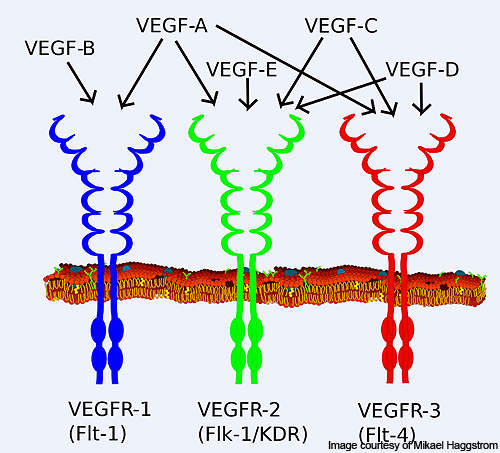
Vascular endothelial growth factor receptor (VEGFR), a signal protein, is responsible for the origination and progression of various tumours. VEGFR-1 plays a major role in the growth of new blood vessels from the old blood vessels (angiogenesis) and tumour growth.
VEGFR-2 plays an important role in the endothelial cell proliferation, migration and survival, and angiogenesis. VEGFR-3 is involved in the formation of new lymphatic vessels from already existing lymphatic vessels (lymphangiogenesis).
Axitinib has the ability to inhibit VEGFR-1, 2 and 3 selectively. It binds to the receptors of the VEGF and inhibits it, thereby preventing the formation of new blood vessels.
Clinical trials of Pfizer’s Axitinib drug
Phase I trials to evaluate the safety, efficacy and pharmacokinetics of the drug were initiated in July 2008. Six patients were enrolled and were administered single doses of 5mg, 7mg or 10mg of the drug.
Phase II trials to study the activity and response rates of the drug in patients with ARCC were initiated in March 2006. Around 62 patients were enrolled and were administered 5mg of Axitinib in 28 day cycles. The primary end point was to obtain overall response rates. The study was completed in February 2009.
The Phase II trial was conducted to evaluate the safety and efficacy of the drug in patients with ARCC. The trial was initiated in August 2009 and around 200 patients were enrolled. The primary end point of the trial was superior overall response rates. The patients were administered 5mg of Axitinib in combination with either Axitinib dose titration or placebo dose titration. The entire study was completed by September 2012.
Phase II trials to determine the objective response rates were initiated in December 2007. A total of 63 patients were enrolled and administered twice daily doses of 5mg Axitinib.
Phase II trials to evaluate the efficacy of the drug were initiated in February 2011. The study will determine the objective response rates of the drug. Around 24 patients have been enrolled who will be administered 5mg of Axitinib orally in twice daily doses for a period of 24 days.
Phase III AXIS 1032, a global trial, was initiated in US, EU and Japan in September 2008 to compare the efficacy and safety of the drug in comparison with sorafenib. It enrolled 723 patients having ARCC who had received prior treatment containing sunatinib (54%), cytokines (35%), bevacizumab (8%) or temsirolimus (3%). The patients were administered either 5mg Axitinib or 400mg sorafenib as twice daily doses. The study was completed by December 2012.
Another Phase III trial was conducted to evaluate the effect of the drug in metastatic renal cell carcinoma patients compared to that of sorafenib. The trial was initiated in August 2009 and completed by December 2011. It enrolled 453 patients and administered them with either 5mg Axitinib or 400mg sorafenib twice daily. The primary end point was superior progression free survival in Axitinib treated patients compared to placebo.
Patients were enrolled for a comparative clinical trial on Axitinib versus placebo in advanced hepatocellular carcinoma patients whom were not cured by anti-angiogenic therapy.
Phase III trial results
The primary trial data of the Phase III study AXIS 1032 was released in May 2010. Patients treated with Axitinib had a superior progression free survival (PFS) period of 6.7 months when compared to the 4.7 months of PFS in sorafinib treated patients. Hence the primary end point was met. Objective response rates more than doubled in Axitinib treated patients when compared to the sorafinib treated patients.
The marketing approvals for Axitinib in the US and Europe were based on the results obtained from the phase III AXIS trial. The results of the study showed that the patients who were treated with Inlyta as the second-line treatment achieved significantly extended progression free survival (PFS) with a median PFS of 6.8 months, when compared to 4.7 months achieved by the patients treated with sorafenib.