Developed by French pharmaceutical company NicOx, naproxcinod (HCT 3012) is the first in a new class of analgesic and anti-inflammatory drugs called COX-inhibiting nitric oxide donators (CINODs). It is indicated for the treatment of acute and chronic nociceptive pain, such as post-operative and arthritic pain.
Following successful completion of Phase III trials, NicOx filed a New Drug Application (NDA) for naproxcinod with the US Food and Drug Administration (FDA) in September 2009. The NDA was based on data from 34 clinical trials involving about 4,000 patients.
In November 2009, the FDA accepted the NDA. On 22 July 2010, NicOx received a complete response letter from the FDA declining the approval of naproxcinod. In the letter, the FDA recommended that additional long-term clinical studies be carried out to assess its gastrointestinal and cardiovascular safety and demonstrate its therapeutic benefits.
NicOx decided to appeal the FDA decision.In July 2011, it initiated the formal dispute resolution process, through which it can request a formal review of the NDA at a higher supervisory level.
NicOx had also submitted a Marketing Authorisation Application (MAA) to the European Medical Agency for Naproxcinod in December 2009. The company, however, withdrew the MAA in April 2011, following negative feedback from the Committee for Medicinal Products for Human Use regarding Naproxcinod’s approval.
CINODs represent a new class of analgesic agents
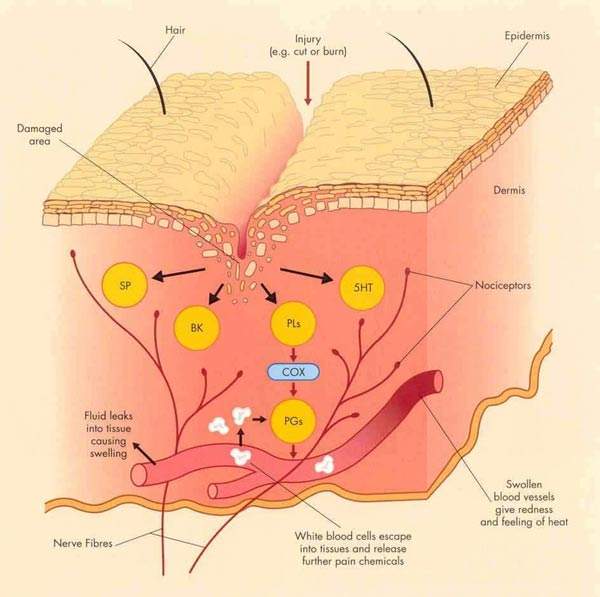
Inhibition of cyclo-oxygenase (COX) enzymes, of which there are two (COX-1 and COX-2), is the basic mode of action of the non-steroidal anti-inflammatory drugs or NSAIDs.
Naproxcinod (HCT3012) is an entirely new chemical entity which provides balanced inhibition of COX enzymes while also donating nitric oxide (NO) at sites of inflammation.
This mode of action may reduce inflammation, as NO is known to exert a relaxing effect on endothelial cells. Donation of NO may also have a protective effect on the gastrointestinal tract and other organs. Damage to the gastrointestinal tract is a known side effect of conventional NSAID use and is believed to be associated with inhibition of COX-1.
Efficacy and safety in acute and chronic pain
Data showing early evidence of the efficacy and safety of naproxcinod (HCT3012) was first presented in 2002. The drug later advanced to Phase III development, where it was investigated in three pivotal trials (301, 302 and 303) designed to investigate its efficacy and safety in patients with osteoarthritis.
Studies 301 and 302 evaluated the safety and efficacy of the drug in patients suffering with osteoarthritis of the knee. The 303 study evaluated the safety and efficacy of the drug in patients suffering with osteoarthritis of the hip.
The 301 study was a 13-week, double-blind, placebo and naproxen-controlled study involving 918 subjects. It was conducted across 120 centres in the US and was completed in October 2006. The 302 study involved 1,020 patients and was conducted at 150 centres across the US. The 53-week, double-blind study was completed in September 2008.
The 303 study was a 13-week trial involving 810 patients. The trial was conducted across 120 centres in the US, Canada and Europe and was completed in November 2008. All three studies reached their primary endpoints and demonstrated good overall safety and tolerability.
OASIS study points to cardiovascular safety
Data from the Phase II OASIS trial, in which six weeks treatment with naproxcinod (HCT3012) proved comparable to the COX-2 specific inhibitor rofecoxib in ameliorating pain in OA patients, also showed no evidence of increases in blood pressure in the naproxcinod (HCT3012) treatment arm. This was in contrast to rofecoxib, which at equi-equivalent doses was associated with a significant increase in systolic blood pressure.
It has been suggested that, via NO release, CINODs may be able to counteract the detrimental effects of COX inhibition on blood pressure. Certainly, concerns have been raised about the cardiovascular safety of COX-2 specific inhibitors. NicOx had initiated a new US study to compare the effects of HCT 3012 with rofecoxib on arterial blood pressure in patients with mild hypertension.
In June 2009, NicOx announced the publication of results of the ZEST Phase II study for naproxcinod. The six-week Phase II trial involving 543 patients evaluated the safety and efficacy of three doses of naproxcinod and one dose of the COX-2 inhibitor rofecoxib in comparison to placebo. The study revealed that there was no statistically significant difference between these three treatment groups.
Marketing commentary
NSAIDs are the cornerstone of analgesia, providing relief from everyday pains such as headache, toothache, muscular aches, mild joint pain and period pain. Although highly effective, they are associated with gastrointestinal side effects and other adverse events.
The need for safer NSAIDs has seen the development of second-generation COX-2 specific inhibitors, with improved gastrointestinal safety. CINODs represent third-generation compounds, with potential to provide improved gastrointestinal and cardiovascular safety.
In March 2011, NicOx signed an agreement with Grupo Ferrer Internacional providing exclusive distribution rights for naproxcinod in Greece and Portugal, if the drug is approved in Europe. The agreement also includes co-marketing rights in Spain and Germany.