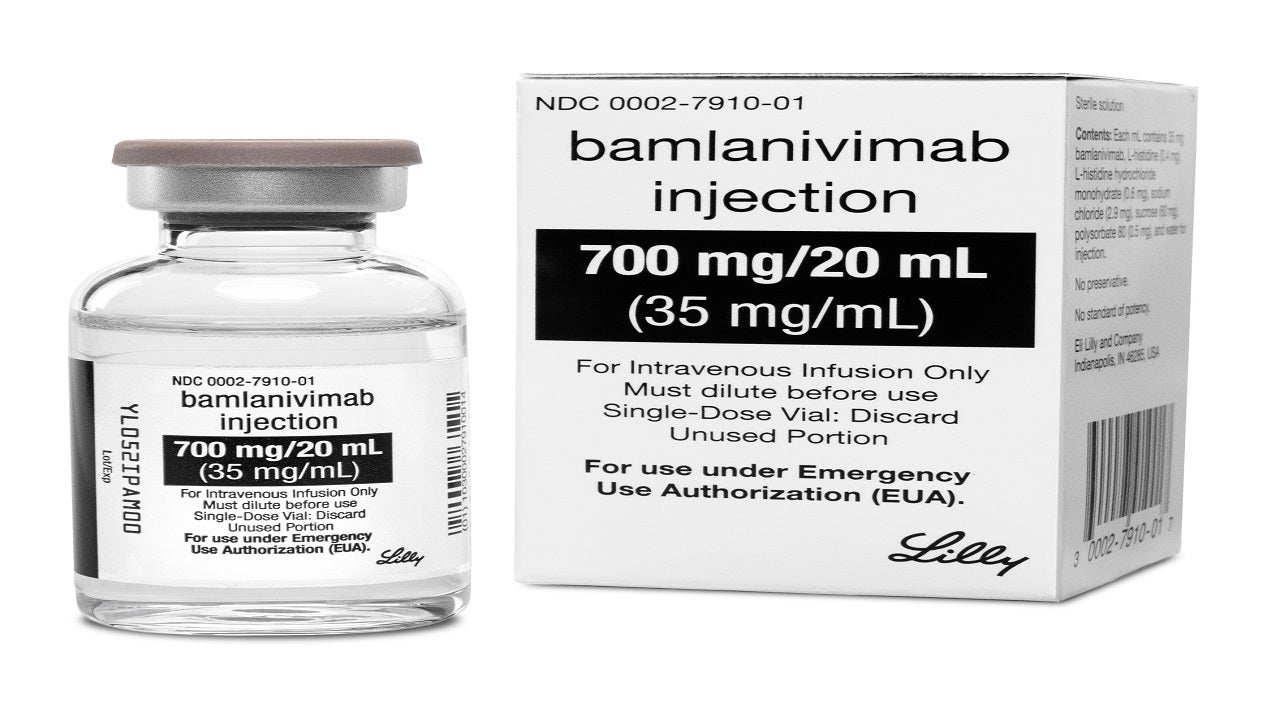
Bamlanivimab (LY-CoV555) is a neutralising monoclonal antibody intended for the treatment of mild to moderate Covid-19.
Developed by Eli Lilly and Company, Bamlanivimab secured emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) in November 2020.
The US government purchased 300,000 doses of bamlanivimab to provide to high-risk patients in the US without any out-of-pocket expense for the drug.
Lilly will ship bamlanivimab to AmerisourceBergen, a national distributor which will distribute the drug according to the US Government’s allocation programme.
Covid-19 test-positive adults and paediatric patients aged 12 years and older, who are at high risk of progression to severe Covid-19 and hospitalisation, are eligible to receive the drug. Bamlanivimab should be administered to the patients promptly, following a positive COVID-19 test and within ten days of onset of symptoms.
Lilly plans to manufacture up to one million doses of bamlanivimab 700mg by the end of 2020 to supply worldwide from early-2021. With the opening of new manufacturing facilities, bamlanivimab’s supply will increase significantly in 2021.
The drug is available as a sterile, preservative-free aqueous solution in 700mg/20mL strength for a single intravenous infusion.
Covid-19 causes and symptoms
Coronavirus disease (Covid-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The Covid-19 outbreak that originated in China was declared a pandemic by the World Health Organisation (WHO) in March 2020.
Coronaviruses are a group of viruses that can cause diseases such as the Middle East respiratory syndrome (MERS), common cold, and severe acute respiratory syndrome (SARS).
Covid-19 spreads mainly by droplets of saliva or discharge from the nose when an infected person coughs or sneezes. Many affected patients experience mild to severe symptoms and recover without hospitalisation, while several patients develop serious or life-threatening complications, including severe respiratory illness and pneumonia.
Covid-19 signs and symptoms can occur two to 14 days after exposure. Early symptoms include loss of taste or smell, while other symptoms are fever, dry cough, shortness of breath, aches and pains, tiredness, diarrhoea, sore throat, headache, conjunctivitis, a rash on the skin, discolouration of fingers or toes, chest pain, and loss of speech or movement.
Bamlanivimab mechanism of action
Bamlanivimab is a recombinant neutralising human IgG1κ monoclonal antibody that binds to the receptor-binding domain of the spike protein of SARS-CoV-2 and prevents the attachment of spike protein with the human ACE2 (a cell surface protein) receptor.
The Covid-19 treatment is unmodified in the fragment crystallizable (Fc) region.
Clinical trials on Bamlanivimab
The emergency use authorisation of Bamlanivimab is based on the interim results of an ongoing randomised, double-blind, placebo-controlled phase two clinical trial, named BLAZE-1, which was performed in 465 non-hospitalised adults with mild to moderate Covid-19 symptoms and tested for Covid-19 based on the sample collected not before three days of infusing the drug.
The patients were randomised to receive a single infusion of 700mg (N=101), 2,800mg (N=107), 7,000mg (N=101) of bamlanivimab or placebo (N=156).
The predetermined primary endpoint of the clinical trial was an improvement in viral load from baseline to Day 11. Most of the patients effectively cleared the viral load by Day 11.
The secondary outcome was Covid-19-related hospitalisations or emergency room visits within 28 days of treatment. Approximately 1% to 2% of the bamlanivimab-treated patients required hospitalisations or emergency room visits within 28 days compared to 6% of patients treated with placebo.
A smaller number of bamlanivimab-treated patients who are at high risk for disease progression (3%) led to Covid-19-related hospitalisations or emergency department visits compared with placebo-treated patients (10%).
The absolute risk reduction for bamlanivimab compared to placebo is greater in patients at higher risk of hospitalisation based on high-risk criteria.
The adverse events observed in patients in the BLAZE-1 trial were vomiting, nausea, diarrhoea, headache, dizziness, and itchy skin.