Benlysta is an investigational human monoclonal antibody drug indicated for treating autoantibody-positive systemic lupus erythematosus (SLE). The drug was jointly developed by Human Genome Sciences (HGS) and GlaxoSmithKline (GSK) under a co-development and commercial agreement.
On 4 June 2010 HGS and GSK applied for European regulatory approval to market the drug. On 10 June HGS submitted a biologics license application to the US Food and Drug Administration (FDA) for the approval of the experimental treatment.
In the same month, Benlysta successfully met its primary endpoint in two pivotal Phase III studies.
On 16 November 2010, 13 out of 15 physicians of an FDA panel voted in favour of Benlysta’s approval. The FDA was expected to announce the drugs approval on 9 December 2010; instead, it requested additional data. The FDA subsequently extended the approval decision date to 10 March 2011.
On 9 March 2011, the FDA approved Benlysta for treating adult patients with SLE. Benlysta is expected to generate annual sales of over $1bn.
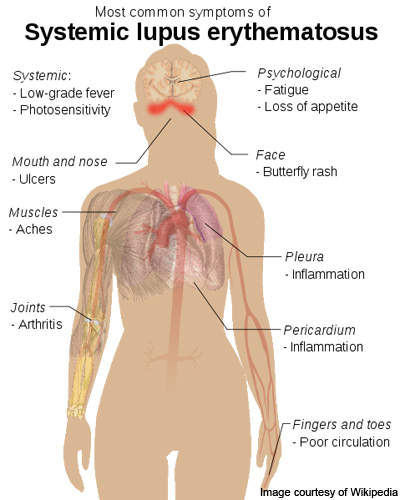
Systemic lupus erythematosus
Lupus is an autoimmune connective tissue disease that can affect any part of the body.
As with other autoimmune diseases, the immune system attacks cells and tissue, causing damage and inflammation. SLE produces antibodies that attack nearly every healthy organ and tissue of the body but it most often targets the skin, blood vessels, lungs, heart, joints, kidneys and the nervous system.
SLE affects more than 1.5 million people in the US and five million people worldwide. The disease occurs nine times more frequently in women than in men and is the leading cause of female premature cardiovascular disease, kidney disease and stroke.
The symptoms of SLE are unpredictable and vary widely, making accurate diagnosis difficult. Scientists have yet to determine what causes the disease and no new drugs have been introduced for 30 years.
Benlysta in action
Benlysta inhibits the biological activity of the B-lymphocyte stimulator (BlyS), which is a naturally occurring protein. BLyS is required for the development and survival of B-lymphocyte cells into mature plasma B cells, which in turn produce antibodies.
Elevated levels of BLyS may contribute to the production of autoantibodies. The results of prospective observational studies show a significant correlation of elevated levels of BLyS with SLE disease activity.
Benlysta recognises and binds to BlyS, inhibiting its stimulation of B-cell development, and restores the potential for autoantibody-producing B cells to enable it to undergo the normal process of apoptosis.
Clinical trials
HGS discovered Benlysta in 1997. It was studied for four years and the results were presented in October 2009 at the ACR Annual meeting. The results of the data showed that Benlysta was associated with the sustained improvement and stabilisation of SLE.
In 2006 Benlysta failed in a mid-stage Phase II trial. However, the data was reexamined and HGS found that Benlysta did appear effective in a subset of patients. Based on that information, HGS and GSK changed the design of the Phase III trial.
HGS designed the Phase III trial in collaboration with GSK and leading international SLE experts. The trial was conducted under a special protocol assessment agreement with the FDA.
The phase includes two double-blind, placebo-controlled, multi-centre superiority trials: BLISS-52 and BLISS-76. The two studies treated 1,684 SLE patients.
In the BLISS-52 trial, 865 patients at 90 clinical sites in 13 countries, including Asia, South America and Eastern Europe, were randomised and treated. The results demonstrated that Benlysta made clinically and statistically significant improvements in patient response rates as measured by the SLE responder index at week 52.
Under BLISS-76, 819 patients at 133 clinical sites in 19 countries were enrolled and randomised.
The BLISS-76 study results, unveiled in October 2010, showed that Benlysta demonstrated higher response rates compared to a placebo. It was also found that the drug continued to be well tolerated and that adverse events rates remained comparable between Benlysta and placebo groups.