Discovered and developed by Novartis, Bexsero is a vaccine indicated for the treatment of meningococcal group B (MenB) disease. It is the first and the only Men B vaccine for the protection of all age groups against the disease.
Novartis received a breakthrough therapy designation for Bexsero from the FDA in April 2014. Novartis submitted Biologic License Application (BLA) to the FDA for the marketing approval of Bexsero in adolescents and young adults from 10 years through 25 years of age in the US in June 2014.
In December 2010, Novartis submitted a Marketing Authorisation Application (MAA) to the European Medicines Agency (EMA) for bexsero based on positive results from Phase III trials.
In November 2012, the Committee for Medicinal Products for Human Use (CHMP) of EMA adopted a positive opinion for approval of the MAA. Novartis received MAA approval for Bexsero from the European Commission (EC) for the prevention of life-threatening meningitis across Europe in January 2013.
Boxsero is also approved in Canada, Australia and 34 other countries. Approximately 30,000 doses of the Bexsero were distributed to the students and staff at the University of California Santa Barbara (UCSB) and Princeton University after four cases of meningitis B were confirmed in November 2013.
Bacterial meningococcal disease
Meningococcal is a life threatening disease which can lead to death within 24 to 48 hours of the first symptoms. The disease manifests in the form of bacterial meningitis, which leads to an infection of the membrane around the brain and spine and a bloodstream infection called sepsis.
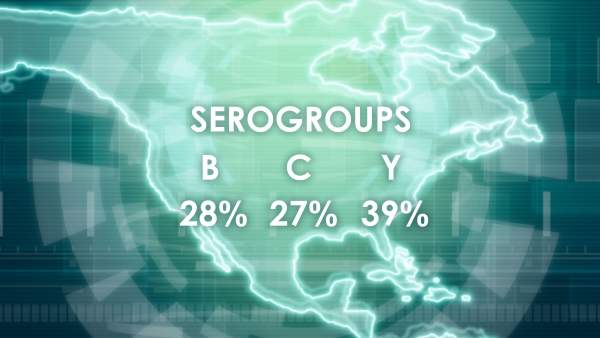
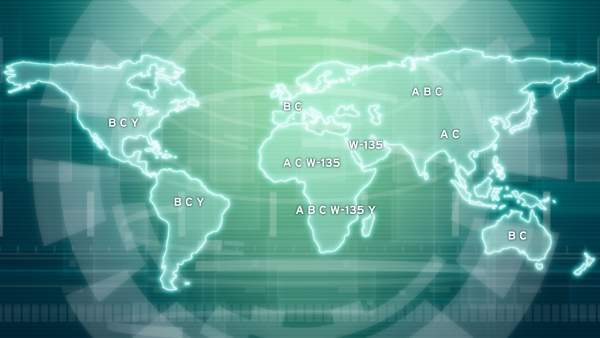
The bacteria which causes meningococcal disease is called meningococcus and is divided into five main groups, called serogroups, namely A, B, C, W135 and Y. MenB is the most common type of bacteria causing meningococcal disease.
MenB strains can mutate making it very difficult to diagnose and treat. It has led to several outbreaks across the world. The highest rates of the disease occur in the semi-arid and sub-Saharan Africa region.
Most of the MenB cases occur in healthy patients. A person can carry the bacteria for up to six months. It is easily transmitted through physical contact, coughing and sneezing. Infants and adolescents are the most vulnerable groups of the disease.
Initial symptoms of the disease are flu-like and hence difficult to diagnose. The main symptoms such as neck stiffness and rashes appear at a later stage of the illness. Existing treatments for the disease include hospitalisation and antimicrobial therapy. The disease, however, is difficult to treat due to its rapid rate of progression.
An estimated 20,000 to 80,000 cases of MenB are reported every year. About 5-10% of the people die even after being diagnosed and treated. Those who survive the disease suffer from severe complications such as brain damage, learning disabilities, behavioural problems and hearing loss.
Bexsero – multicomponent meningococcal serogroup B vaccine
Bexsero has been developed using reverse vaccinology. The technology decodes the genome sequence of MenB and selects proteins which are the most broadly-effective vaccine candidates.
Scientists at Novartis examined nearly 580 potential proteins which can kill the bacteria.
Four distinct antigen components, including factor H binding protein variant 1.1 (fHBP 1.1), neisseria meningitidis adhesin A (NadA), neisseria heparin binding antigen (NHBA) and PorA, have been selected.
These components are immunogenic independently but together provide protection against a range of disease-causing strains.
Novartis used a predictive model called meningococcal antigen typing system (MATS) to assess the potential of the vaccine. The technology helps in predicting the effectiveness of a vaccine. Using MATS, predictions were made as to whether different strains of bacteria were killed by the vaccine.
Clinical trials and marketing commentary for Novartis’s treatment
Bexsero was tested in more than 7,500 patients including infants, toddlers, adolescents and adults across the world.
In September 2010, a Phase III trial conducted in 3,600 infants met its primary end points. The study indicated that most of the infants administered with bexsero developed a strong immune response against all antigens. An extension of this study also met its primary end points.
Results from a Phase II-b / III study involving 1,800 infants and toddlers were announced in June 2011. The results indicated that bexsero developed a robust immune response in infants.
About 1,631 adolescents aged between 11 and 17 years old were enrolled for an observer-blind Phase III study. Bexsero was well tolerated and developed robust immune response in patients.
Phase II studies conducted in adults indicated that the drug was well tolerated.
Vaccines targeting meningococcal disease caused by serogroups A, C, W135 and Y are already available in the market. Treatment for MenB, however, still remains unavailable. The market for MenB treatment therefore represents huge potential.