Elacytarabine (CP-4055) is a lipid-conjugated version of cytarabine, an anti-cancer agent drug approved for the treatment of blood-related cancers or haematological malignancies, commonly used to treat patients with leukaemia.
Elacytarabine is being developed by Oslo-based Clavis Pharma and is currently in phase III development for acute myeloid leukaemia (AML). The drug has received Orphan Drug designation from the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The designation provides Clavis Pharma with exclusive marketing rights for seven years in the US and ten years in the EU.
In December 2010, elacytarabine received Fast Track designation from the FDA for the treatment of AML. The designation will provide elacytarabine with a priority review of six months instead of the standard ten months.
Acute myeloid leukaemia
AML is a type of cancer that develops in the bone marrow. In a healthy person, the bone marrow consists of a small quantity of immature blood cells known as blast cells. These cells mature and develop into white blood cells, red blood cells and platelets, which are later released into the bloodstream.
In people suffering from AML, there is an abnormal production and maturation of blast cells or leukaemic blasts in the bone marrow. The leukaemic blasts rapidly increase in number, accumulate in the bone marrow and block the production of normal blood cells.
A decrease in the number of normal blood cells makes people with leukaemia more vulnerable to diseases and infections.
The leukaemic blasts also tend to spread from the bone marrow to other parts of the body such as lymph nodes, liver and the central nervous system. Symptoms of AML include fatigue, infections, anaemia and excessive bleeding. AML can progress rapidly and needs immediate medical attention after being diagnosed.
It is estimated that there were 20,000 new cases of AML in the US in 2010 and the disease mainly affects patients over 60 years old. Despite progress in the treatment of AML, fewer than 10% of patients in this age group survive beyond five years, and long-term survival in younger adults is 30%.
Elacytarabine (CP-4055)
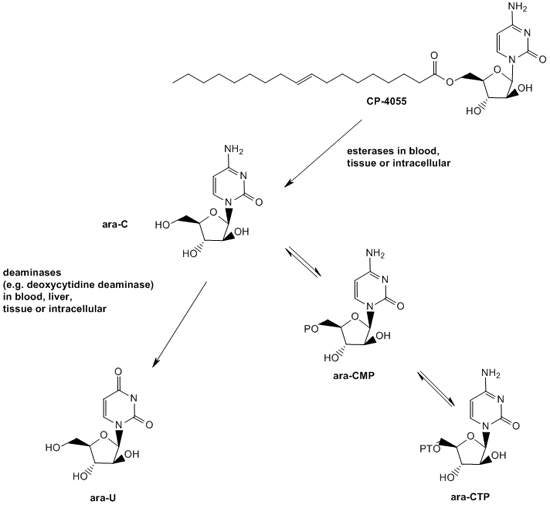
Elacytarabine was invented using Clavis Pharma’s Lipid Vector Technology. The drug is a fatty acid derivative of cytarabine and belongs to the nucleoside analogue class.
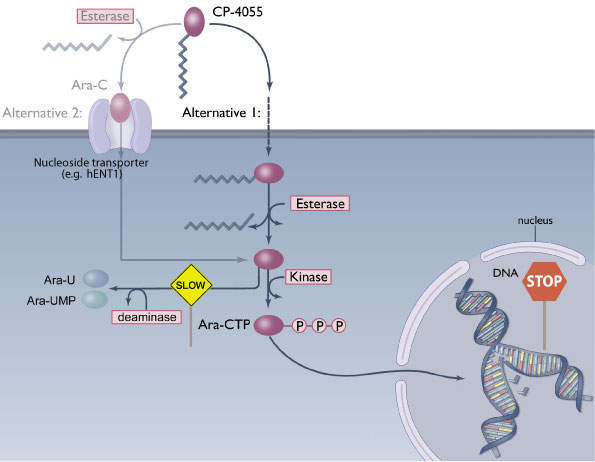
Cytarabine is used in approximately two thirds of patients with AML and is also used to treat other haematological malignancies. For entry into cells, cytarabine is dependent on the human equilibrative nucleoside transporter (hENT1), a transmembrane protein. Once inside the cells, the drug acts by interfering with DNA replication and stops cell division with rapidly dividing cells such as leukaemia cells being most affected.
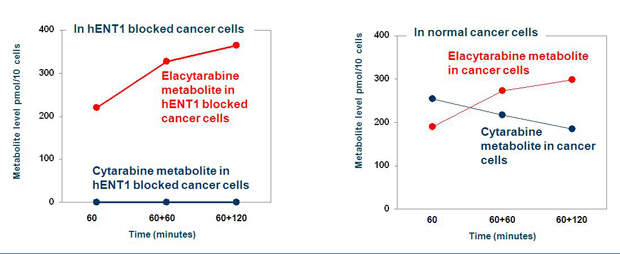
Cytarabine has a limitation. It has a minimal uptake in tumour cells, especially in AML cells that have low expression levels of hENT1. The drug is, therefore, less effective in such AML patients. Elacytarabine can enter into leukaemia cells independent of hENT1.
Clinical trials
The phase I trial of elacytarabine concluded in September 2004. The trial included 24 patients suffering from advanced malignant melanoma, lung cancer or ovarian cancer. Elacytarabine was administered as monotherapy in these patients. Results of the phase I trial established elacytarabine’s safety profile.
Based on the data of the phase I trial, Clavis Pharma advanced elacytarabine into phase II trials. The first phase II trial concluded in 2009 and recruited 61 late stage AML patients who did not respond or in whom the disease relapsed after two separate rounds of treatments. The trial indicated that elacytarabine significantly improved the median survival period in late stage AML patients to 5.3 months compared with 1.5 months in available clinical data.
The second phase II trial in early stage AML patients is currently recruiting. The study will enrol 50 patients in ten centres across the US and Europe. These patients will be administered elacytarabine in combination with idarubicin and the hENT1 level will be measured. The aim of the trial is to establish that elacytarabine is independent of hENT1 levels in patients. Results from the trial are expected in the third quarter of 2011.
Elacytarabine has been advanced into phase III trials with the CLAVELA study in patients suffering with late-stage AML. The open-label, randomised, controlled trial will enrol 350 people across 65 centres in the US, Europe and Australia. Clavis Pharma enrolled the first patient in the trial in August 2010. Patient enrolment is scheduled to conclude by the end of 2011.
Results of the CLAVELA study are expected in the second half of 2012. If the results are positive, Clavis Pharma is planning to file an NDA and MAA in the same year.
Marketing commentary
With 300,000 new cases of leukaemia (all types) being recorded annually across the world, there is huge demand for new drugs to treat the disease.
Leukaemia is known to be responsible for 220,000 deaths annually. In addition, the recurrence rate of the disease is very high.
Global sales of cytotoxic cancer drugs such as cytarabine are estimated at $10.5bn. Considering the market need for newer drugs, elacytarabine could generate significant revenues for Clavis Pharma.