Bristol-Myers Squibb’s (BMS) ixabepilone (epothilone B) is a new chemotherapeutic agent that is currently in development for the treatment of advanced breast cancer.
In June 2007, the company filed an NDA with the US Food and Drug Administration (FDA) for the use of ixabepilone in patients with metastatic or locally advanced breast cancer. Granted priority review by the FDA, ixabepilone was approved in October 2007 as a new treatment for metastatic or locally advanced breast cancer.
Ixabepilone, which is marketed under the brand name Ixempra in the US since October 2007, is indicated for use in combination with capecitabine in patients who no longer benefit from chemotherapy with anthracyclines (such as doxorubicin or epirubicin) and taxanes (such as paclitaxel or docetaxel). It can also be used alone in patients who no longer benefit from an anthracycline, a taxane and capecitabine.
Following the drug’s launch in the US, BMS submitted new drug applications (NDA) in the European and Japanese markets in September and December 2007 respectively. The European NDA was withdrawn after the Committee for Medicinal Products for Human Use (CHMP) in Europe issued a negative opinion in November 2008.
BMS has reached a collaborative agreement with Otsuka Pharmaceutical, under which Otsuka is to sell Ixempra on a regressive tiering basis on behalf of BMS from 2010 to 2020.
Targeting microtubules in breast cancer
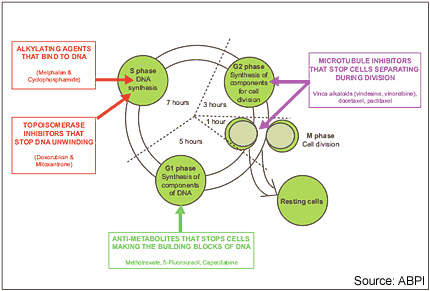
Drugs that disrupt microtubule formation in tumour cells, so-called microtubule inhibitors, represent an important group of anti-cancer agents. Established classes of microtubule inhibitors include the vinca alkaloids and the taxanes. These drugs disrupt microtubules involved in cell separation during the G2 phase of the cell cycle.
Taxanes include BMS’ Taxol (paclitaxel), a drug that is considered a valuable component of treatment regimens for patients with breast cancer in both advanced and early-stage disease.
Ixabepilone is similar to paclitaxel in targeting cell microtubules and the β -tubulin subunit in particular, but is structurally quite different to the taxanes. Ixabepilone is an analogue of epothilone B.
Epothilones are a new class of microtubule inhibitors, which were isolated initially from the fermentation of the cellulose degrading soil bacterium Sorangium cellulosum. By binding to tubulin, epothilones cause mitotic arrest and resultant cell
death or apoptosis.
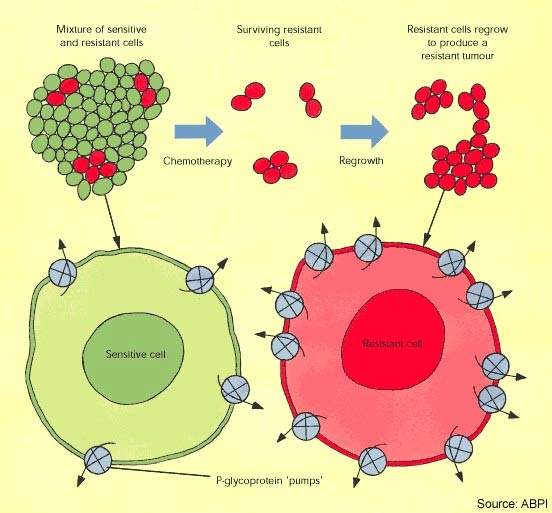
Combating drug resistance
Cytotoxic drugs have proved potent weapons in the fight against malignant tumours and are considered first-line therapy for the treatment of many cancers. However, while patients often respond well to a first course of chemotherapy, over time the response to drug treatment diminishes and the tumour may eventually become drug resistant.
In some cases resistance can develop across several classes of anti-cancer drugs, leading to multidrug resistance. The development of drug resistance limits the effectiveness of many anti-cancer agents and is an important contributor to cancer deaths.
Although taxanes, such as paclitaxel, are among the most active anti-cancer agents against a wide range of solid tumours, drug resistance is a well-known problem with this class of drugs. A majority of cancer patients initially responsive to treatment with taxanes will eventually develop resistance.
Epothilones appear far less susceptible to tumour resistance mechanisms known to affect the taxanes. These include overexpression of P-glycoprotein, β -tubulin mutations and high microtubule-associated protein tau expression.
Clinical studies suggest that epothilones are active in breast cancer patients whose disease has progressed while on taxanes. This makes epothilones, such as ixabepilone, a potentially valuable new class of anti-cancer agents for patients with taxane-resistant cancer.
Clinical trials support use of ixabepilone in breast cancer
In December 2008, data from two Phase III clinical trials of the drug involving about 2,000 patients was presented at the San Antonio Breast Cancer Symposium (SABCS). Data from the pooled analysis of the subset of 443 patients with triple negative breast cancer indicated that Ixempra plus capecitabine was the first combination regimen to show statistically significant PFS compared to capecitabine alone.
The overall response rate was 31% compared to 15% for patients who were administered capecitabine alone. The new data demonstrated the effectiveness of Ixempra plus capecitabine in improving progression-free survival in triple negative metastatic breast cancer patients.
The efficacy of ixabepilone was demonstrated in a series of clinical trials in which the drug was administered either alone (monotherapy) or in combination with capecitabine to patients with metastatic breast cancer. Overall the data indicate that in this difficult-to-treat population, ixabepilone can induce measurable tumour responses and increase progression free survival.
In 49 patients with taxane-resistant metastatic breast cancer, 12% partially responded to treatment with ixabepilone. Among responders in this study, median response duration was 10.4 months; median time to progression was 2.2 months and median survival 7.9 months.
Ixabepilone also proved effective in a study of 23 taxane-naïve patients with metastatic breast cancer, where 57% achieved a partial response and 26% stable disease while on ixabepilone therapy.
Of 126 patients with advanced breast cancer resistant to anthracyclines, taxanes and capecitabine, treatment with ixabepilone produced a response in 18% of patients, with 50% having stable disease.
Results from a 752-patient study, in which patients with anthracycline- and taxane-resistant breast cancer received ixabepilone plus capecitabine or capecitabine alone, showed that combination therapy was more effective than capecitabine alone; progression-free survival was 5.8 and 4.2 months respectively.
In addition to the 40% increase in progression-free survival, more than twice as many patients achieved an objective tumour response in the combination treatment arm compared with those on capecitabine alone (35% vs. 14%).
Side effects are an acknowledged problem with cytotoxic agents, with neurotoxicity a known side effect of tubulin-active drugs. In the ixabepilone trials in breast cancer patients, adverse events included treatment-related neuropathy.
Marketing commentary
Although there have been major advances in the treatment of breast cancer in the last ten to 15 years, it remains a disease for which additional treatments are still needed to improve outcome. The epothilones represent a potentially important new class of anti-cancer drugs, especially for patients who develop resistance to standard therapies. In addition to potent anti-tumour activity, ixabepilone is seen as clinically attractive because of its complete lack of cross-resistance with commonly used drugs including taxanes.