Cabenuva is a complete antiretroviral regimen comprising Vocabria (cabotegravir) and Edurant (rilpivirine) for the treatment of HIV-1 infection in adult patients with virologically stable and suppressed viral load.
The drug was developed by ViiV Healthcare, a joint venture between GlaxoSmithKline (GSK), Pfizer and Shionogi.
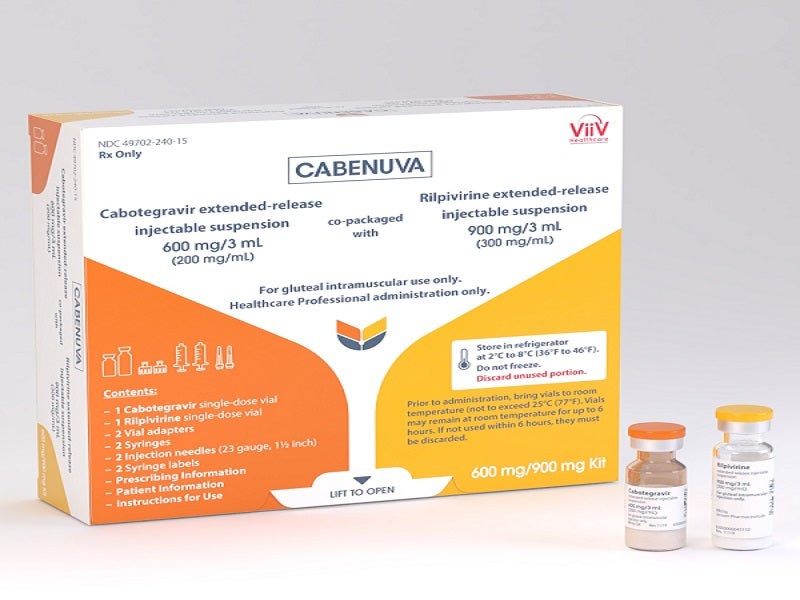
Cabenuva is a long-lasting extended-release (ER) injectable suspension, the complete dose of which is administered with two gluteal intramuscular injections, one for cabotegravir and the other for rilpivirine.
The drug is available as 2ml and 3ml dosing kits with two injectable medicines. Each kit comprises cabotegravir as a white to light pink, free-flowing ER injectable suspension in 200mg/ml strength and rilpivirine as a white to off-white ER injectable suspension in 300mg/ml strength.
Cabotegravir approvals
In April 2019, ViiV Healthcare submitted a new drug application for a two-drug regimen of cabotegravir and rilpivirine to the US Food and Drug Administration (FDA). The company also filed a marketing authorisation application (MAA) for the combination with the European Medicines Agency (EMA) in July 2019.
In December 2019, ViiV Healthcare received a complete response letter from the FDA. The letter cited safety concerns related to chemistry manufacturing and controls, as well as the safety profile of the drugs.
The FDA granted breakthrough therapy designation to cabotegravir for HIV pre-exposure prophylaxis in November 2020. Cabenuva was also granted fast track and priority review designation by the FDA.
Health Canada approved Cabenuva in March 2020. The drug has also been approved by the regulatory authorities in Switzerland and Australia.
In December 2020, the EMA approved the MAA for the long-acting regimen of cabotegravir and rilpivirine for use every two months as Vocabria and Rekambys.
The FDA approved Cabenuva as a once-monthly, complete regimen for the treatment of HIV-1 infection in January 2021.
In February 2022, the FDA approved Cabenuva for dosing every two months for the treatment of HIV-1 in virologically suppressed adults.
The following month, the FDA approved an expanded indication for the drug for treating HIV-1 in virologically suppressed adolescents aged 12 years or older and weighing at least 35kg.
Cabenuva received approval from Health Canada in March 2020 and is also approved by the regulatory authorities in Switzerland and Australia.
HIV causes and symptoms
Human immunodeficiency virus (HIV) destroys cells that help the body combat infection, making it more susceptible to other pathogens and diseases.
The disease spreads through the infected body fluids of a person infected with HIV, most often through either casual intercourse (sex without a condom or measures to avoid or treat HIV) or through the sharing of injection drug equipment. If left untreated, HIV leads to AIDS (acquired immunodeficiency syndrome) disease.
Symptoms of the disease include fatigue, chills, rash, mouth ulcers, night sweats, muscle aches, fever, swollen lymph nodes, and sore throat.
Cabenuva’s mechanism of action
Cabotegravir acts as an integrase strand transfer inhibitor. It blocks HIV integrase by attaching to the active integrase site and inhibiting the retroviral deoxyribonucleic acid (DNA) integration stage, which is necessary for the HIV replication cycle.
Rilpivirine is a diarylpyrimidine non-nucleoside reverse transcriptase inhibitor of HIV-1.
Clinical trials on Cabenuva
Health Canada’s approval of Cabenuva was based on the results of two pivotal Phase III clinical studies, Antiretroviral Therapy as Long-Acting Suppression (ATLAS) and First Long-Acting Injectable Regimen (FLAIR). The two trials together enrolled more than 1,100 patients from 16 countries.
The drug safety profile was based on 48-week analyses of pooled data from the two trials.
Patients previously treated with antiretroviral regimen were enrolled in ATLAS, while those enrolled in FLAIR were antiretroviral treatment-naive but had previously received a regimen containing dolutegravir (INSTI) for 20 weeks.
In clinical studies, randomised patients received either Cabenuva or remained on their antiretroviral regimen. Patients receiving Cabenuva intramuscularly once a month showed similar efficacy to those continuing their daily, oral antiretroviral regimens to maintain viral suppression throughout the 48 weeks.
Around 88% of patients preferred Cabenuva, while just 2% preferred their previous antiretroviral regimen in a single-item question survey performed in 532 patients at week 48.
The most common adverse events reported in patients in the two studies were injection site reactions, high body temperature, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, rash, and diarrhoea.
The FDA approved long-acting Cabenuva for use every two months based on the results of the worldwide ATLAS-2M phase IIIb trial, an ongoing, randomised, open-label, active-controlled, multicentre, parallel-group trial, which enrolled 1,045 adults living with HIV-1.
The ATLAS-2M is designed to evaluate the non-inferior antiviral activity and safety of long-acting cabotegravir and rilpivirine long-acting regimen. The results of the study showed that one dose every two months was not inferior to once-monthly dosing.
The FDA’s approval of Cabenuva in virologically suppressed adolescents was based on results of the MOCHA (IMPAACT 2017) study, a Phase I/II, multi-centre, open-label, non-comparative study in 23 virologically suppressed adolescents aged between 12 and 18 years.
The study examined the safety, tolerability and acceptability of the drug in adolescents living with HIV-1. Results from the study showed that the drug’s safety and efficacy were similar to those established in cabotegravir plus rilpivirine administered in adults.