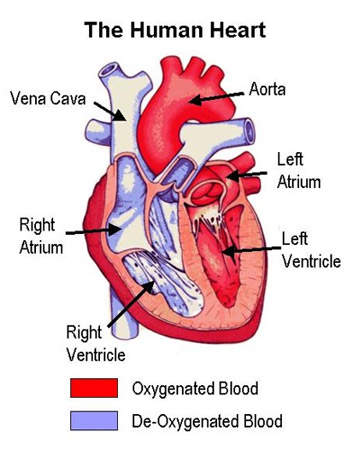
TRA-SCH 530348 is an oral antiplatelet drug under development by Schering-Plough for the treatment and prevention of atherothrombotic events in patients with acute coronary syndrome (ACS), previous myocardial infarction (MI), stroke, or existing peripheral arterial disease.
On the back of successful Phase II clinical trials, TRA-SCH 530348 has now progressed to Phase III development, where it is being evaluated in two large-scale trials involving almost 30,000 cardiac patients.
The drug was awarded fast-track status by the US Food and Drug Administration to speed up the review of the drug.
Strategies for preventing atherothrombotic events
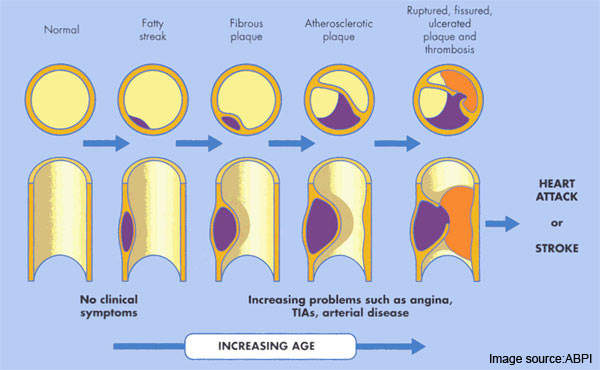
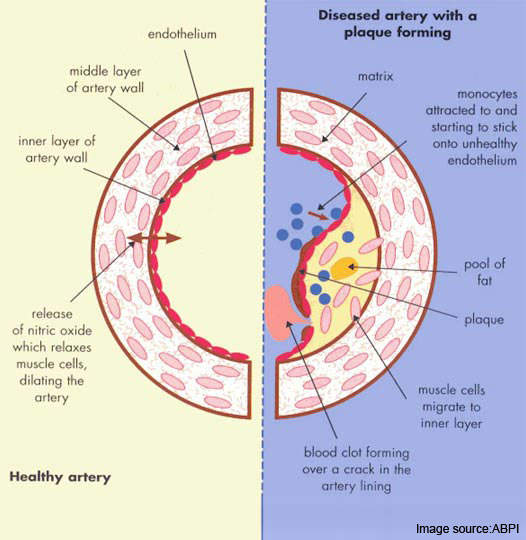
Atherosclerosis is a pathological process in which arteries supplying the heart become progressively narrowed by the build up of fatty material in the artery wall. Known as atherosclerotic plaques, they lead to narrowing or stenosis of the artery lumen so restricting blood flow to the heart.
The resulting lack of oxygen to heart muscle (myocardial ischaemia) leads to adverse clinical consequences such as angina, myocardial infarction and sudden cardiac death.
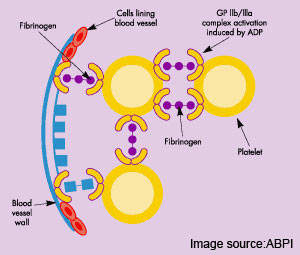
Most acute cardiovascular events, such as MI, occur when platelets are activated at the site of atherosclerotic plaque rupture and then release substances that lead to platelet aggregation and blood clot formation (thrombosis).
A thrombosis, which can result in partial or complete blockage of an artery, is responsible not only for ACS but also for other vascular events such as ischaemic stroke, another major cause of death and disability.
Antiplatelet drugs, such as aspirin and clopidogrel, are an important component of preventive medical therapy to reduce the risk of further occlusive vascular events in at-risk patients. However, there remains a need for additional antiplatelet drugs that can help further reduce the risk of cardiovascular and cerebrovascular events without increased risk of bleeding.
Thrombin-receptor antagonists – a new class of antiplatelet drugs
Unlike currently available antiplatelet drugs, TRA-SCH 530348 is designed to prevent blood clots by inhibiting the action of thrombin, a key coagulation factor that drives the clotting process by converting fibrinogen into fibrin – the final step in blood coagulation. TRA-SCH 530348, the first in a new class of drugs called thrombin-receptor antagonists, blocks the platelet PAR-1 receptor to which thrombin binds. It thus prevents thrombin-induced activation of platelets and subsequent
thrombus formation.
TRA-SCH 530348 advances to Phase III development
Following promising results from Phase II trials in patients undergoing PCI, in which the addition of TRA-SCH 530348 to standard antiplatelet therapy showed a trend towards reduced incidence of ischaemic events, TRA-SCH 530348 has advanced to pivotal Phase III studies.
In these randomised, placebo-controlled trials, TRA-SCH 530348 will be evaluated as a treatment for patients with ACS and, in TRA 2P-TIMI 50, for secondary prevention in patients who have had a prior MI or stroke or have
peripheral artery disease. The patients receive 2.5mg tablets once a day.
Involving approximately 10,000 subjects, the ACS trial will follow patients over a year and assess the impact of therapy with respect to cardiovascular mortality, MI, re-hospitalisation for ACS, urgent coronary revascularisation and stroke (composite primary endpoint).
The secondary trial involving approximately 19,500 patients will assess the effects of a maintenance dose of TRA-SCH 530348 on the frequency of cardiovascular mortality, MI, re-hospitalisation for ACS, urgent coronary revascularisation and stroke (composite primary endpoint). In both trials all patients will receive standard antiplatelet therapy with aspirin and clopidogrel.
The Phase III trial will be carried out until 2,279 primary end points (composite of cardiovascular death, myocardial infarction and stroke) and 1,400 secondary end points (composite of cardiovascular death and myocardial infarction) are recorded. The primary trial is expected to be completed by September 2010 or early 2011.
Marketing commentary
Coronary heart disease (CHD), the physical manifestation of atherosclerosis, is a major cause of death and disability. Of the 17 million deaths that occur from cardiovascular disease in the world each year, CHD is a significant contributor. Patients with CHD often have atherosclerosis in other vascular beds, predisposing them to the risk of other occlusive events such as ischaemic stroke.
Antiplatelet drugs, together with antihypertensive and lipid-lowering agents, form part of the panoply of drugs that are used in the long-term management of patients who have experienced an episode of ACS. Schering-Plough’s TRA-SCH 530348 has potential to expand the range of antiplatelet therapies.