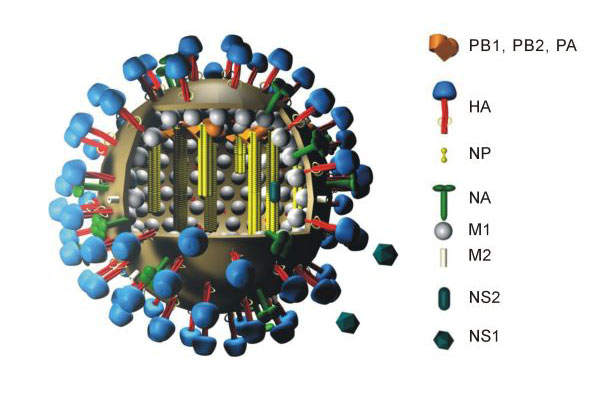
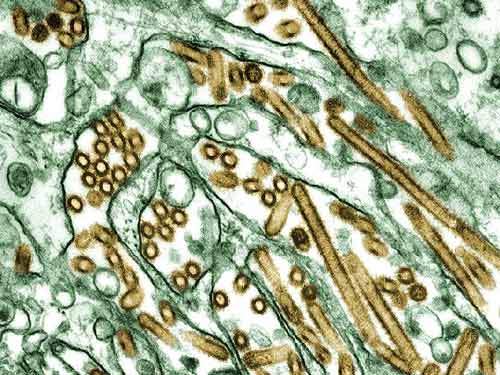
The Celvapan pandemic vaccine is being developed by Baxter International. It was first developed as a mock-up vaccine containing the H5N1 strain of the flu virus called A/Vietnam/1203/2004.
Celvapan is the first vero cell technology-based influenza vaccine to receive marketing authorisation in the EU. This vaccine induces protection against the H5N1 strain influenza virus.
Targeting pandemic influenza
The European Medicines Agency (EMEA) approved a mock-up licensure for Baxter’s pandemic vaccine that applies for the change in the strain with the new influenza A/California/07/2009 (H1N1)v virus strain in producing the pandemic vaccine.
This mock-up vaccine with the H1N1 strain showed protective levels of antibodies in about 90% of the people who were studied. This change of strain did not alter the characteristics of the vaccine and the Committee for Medicinal Products for Human Use (CHMP) was satisfied with the results.
The main side effect of this vaccine is pain at the site of injection. The vaccine received marketing authorisation from the CHMP based on positive protective levels over its side effects. However, the information obtained on the pandemic vaccine is not complete and will be reviewed every year by the EMEA.
In May 2009, Baxter received the A/H1N1 strain for testing and evaluation from the US Centers for Disease Control and Prevention. In June 2009, the pre-production and evaluation testing of the virus strain in the vero cell culture was completed.
On 3 June 2009, Baxter initiated commercial production and made its first commercial product just 12 weeks after receiving the virus strain. Celvapan is being produced at one of the world’s largest cell culture vaccine production facilities in Bohumil, Czech Republic. The vaccine is sent to Vienna, Austria for final formulation, filling and finishing before distribution.
In July 2009, the license application for Celvapan was initiated by Baxter based on the EMEA’s guidelines for pandemic vaccine marketing authorisation. On 7 October 2009, Baxter confirmed that the European Commission granted marketing authorisation for the vaccine. Celvapan is being developed to be used as a vaccine to protect against pandemic H1N1 influenza.
Clinical trials underway
The EMEA has already granted mock-up licensure for Celvapan using the H5N1 strain with pandemic potential, based on clinical trials on over 1,300 people.
Clinical trials to evaluate the safety and immunogenicity of Celvapan H1N1 in adults, the elderly and children are underway. The clinical trials are being conducted to obtain the immunogenicity and safety data at dose levels of 7.5µg and 3.75µg of the H1N1 pandemic influenza vaccine in 400 healthy adults of 18 years or older. The trials started in August 2009 and are expected to be complete in November 2009.
In September 2009, clinical trials to study and determine the preferred dose of H1N1 in a paediatric population, in 400 children and adolescents aged six months to 17 years, began. The estimated completion date of the study is May 2010, with an estimated primary completion date of October 2009.
In October 2009, an observational study was started to estimate the incidence of any medically attended adverse events in the vaccinated subjects. The study will be conducted on 9,000 people of different age groups, including children.
The primary safety data in adults and elderly people has indications of good tolerance of the vaccine. The dosage levels are also being tested to confirm whether a single or double dose is required for protection from the pandemic.
Marketing commentary
The World Health Organization (WHO) declared H1N1 to be pandemic and on 12 June 2009 raised the alert level to six.
Meanwhile, Baxter has completed its first commercial product against H1N1 and is ready to start distribution after obtaining the necessary authorisations.
In early October 2009, the Celvapan H1N1 pandemic vaccine received positive opinion from the CHMP of the EMEA. In the same month, Celvapan was granted marketing authorisation by the CHMP.
Baxter has been delivering initial quantities of Celvapan to national public health authorities that have pandemic agreements, including the UK and Ireland.