Cerovive, a nitrone-based free radical scavenger indicated for the treatment of acute ischaemic stroke has been discontinued after the SAINT 2 (Stroke-Acute Ischemic NXY Treatment) trial. It acted as a neuroprotectant, preserving neuronal tissue at risk of dying in the aftermath of stroke. It was developed by Renovis, a US biotechnology company, and licensed to AstraZeneca.
Cerovive had been in phase III development where it was evaluated in over 3,000 stroke patients in a series of clinical trials. Regulatory filing, anticipated in the second half of 2006, awaited the outcome of the full phase III development programme.
Although some encouraging results had been seen in the SAINT 1 trial, this was not substantiated in the subsequent SAINT 2 trial and a decision was taken to cease further development of cerovive as a treatment for ischaemic stroke.
Ischaemic stroke
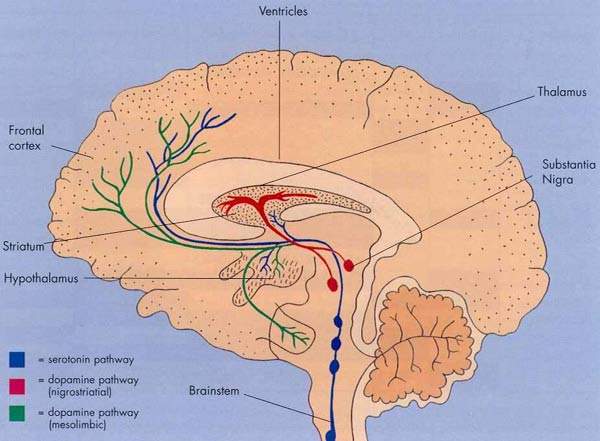
An ischaemic stroke occurs when there is an interruption in the supply of blood to the brain, while a haemorrhagic stroke occurs when a cerebral artery bursts causing bleeding into the tissues of the brain. The loss of blood flow to the brain that follows an ischaemic stroke leads to brain cell injury and death. A fluid-filled cavity or infarct gradually replaces the dead brain cells in the stroke-damaged area.
Neuroprotective agents that might save brain cells from injury following an ischaemic stroke are not yet available. Thrombolytic tPA (tissue plasminogen activator) is the only approved drug for the treatment of ischaemic stroke. However, to be effective it needs to be administered within the first three hours of a stroke. Because it can be damaging to patients with hemorrhagic stroke, screening is needed to establish the aetiology of stroke before tPA administration. This can seriously delay the start of tPA therapy.
The dearth of drugs for treating stroke, a major cause of death and disability, makes this an area of substantial unmet need for new effective drug treatments. Drugs are not the only treatment gap. There is also a need to devise schemes to get stroke victims to hospital sooner.
Currently, this is a big obstacle to expanded use of t-PA and is also likely to be an issue with other new drugs that need to be administered soon after the onset of stroke.
NMDA receptor antagonists
Stroke has proved one of the most difficult diseases to treat effectively. The field is littered with high profile and expensive failures, often in late-stage development.
Interestingly, of the nearly 40 drugs that failed most were closely related to a class of compounds called NMDA receptor antagonists. Large amounts of the neurotransmitter glutamate are released during a stroke or seizure. This causes excess stimulation of glutamate receptors, such as the N-methyl-D-aspartate (NMDA) glutamate, in the brain and neuronal death.
Blocking the NMDA receptor was seen as a valid target. However, many investigational NMDA receptor antagonists had a narrow therapeutic index. This resulted in unacceptable side effects at clinically effective doses.
Compared to other disease states stroke research is still in its infancy. Nonetheless there is a growing understanding of the physiology, vasculature and genetics of ischaemic stroke, enabling the identification of new therapeutic targets. The clinical need is also too large to ignore.
Cerovive was expected to meet all the guidelines established externally for selecting compounds to tackle stroke and different from anything that had been tested before. It was thought that new generation compounds such as cerovive, combined with better-designed preclinical and clinical trials, might stand a much better chance of succeeding than their predecessors.
Cerovive efficacy not sustained
In a primate model of stroke, administration of cerovive produced substantial functional improvements as well as a reduction in infarct volume. Subsequent phase II clinical studies showed good toleration at the doses which produced significant efficacy in the primate models.
In the phase III development programme, cerovive was evaluated in the two international SAINT trials, as well as in the CHANT (Cerebral Hemorrhagic and NXY-059 Treatment) trial, a global safety study of approximately 600 subjects with acute haemorrhagic stroke.
The SAINT trials were designed to compare the effects of cerovive with placebo on disability (loss of sight, impaired speech) and neurological recovery following stroke.
Although SAINT I had hinted at some efficacy, with a statistically significant reduction versus placebo on the primary outcome of disability after acute ischemic stroke (p= 0.038), as measured by the modified Rankin Scale (mRS), the larger SAINT 2 trial was negative for all endpoints. After SAINT 1, enrolment in SAINT 2 was raised from 1,700 to 3,200 patients to increase the trial’s statistical power.
Marketing commentary
After heart disease and cancer, stroke is the third leading cause of death in western industrialised countries and the major cause of adult disability. Cost of ischemic stroke in the US in the next half century is expected to exceed $2.2t.
Attempts to develop effective neuroprotectants for stroke victims have proved difficult and the market opportunities for a company that successfully overcomes the therapeutic hurdles are enormous.
Despite the setbacks seen with cerovive and the decision to stop further development, researchers are anxious that work on neuroprotectants continues. An important outcome of the cerovive trials has been the recognition that pharmaceutical companies need to do at least two adequately powered phase III trials that show consistent results, especially in subgroup analyses, before regulatory authorities grant approval.