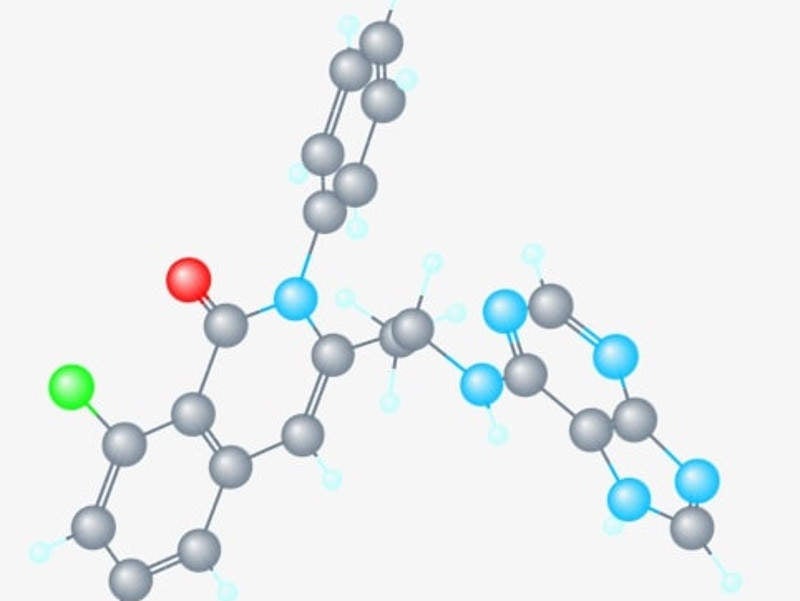
COPIKTRA (duvelisib) is a dual phosphoinositide 3-kinase (PI3K) inhibitor indicated for the treatment of patients with chronic lymphocytic leukaemia (CLL), small lymphocytic lymphoma (SLL) or follicular lymphoma (FL).
The drug was initially developed by Infinity and licensed by Verastem in December 2016. It is one of the first delta-gamma inhibitors to be approved by the US Food and Drug Administration (FDA).
The FDA accepted a new drug application (NDA) for the drug under priority review in April 2018 and subsequently approved it for the treatment of third line CLL/SLL in October 2018. The drug was also granted orphan drug and fast track designations for CLL, SLL and FL.
A European marketing application is planned to be submitted by the end of 2018.
Chronic lymphocytic leukaemia and small lymphocytic lymphoma causes and symptoms
CLL and small lymphocytic lymphoma are two different forms of cancer. CLL is characterised by abnormal production of lymphocytes in the bone marrow that prevents the production of healthy white blood cells, red blood cells and other components of the blood. The abnormal lymphocytes are also known as leukaemia cells. In SLL and FL, cancerous cells are found mostly in the lymph nodes.
The symptoms of CLL include swollen lymph nodes, tiredness, a swollen abdomen, fatigue, shortness of breath, anaemia, bruising easily, weight loss and frequent infections.
An estimated 200,000 patients a year in the US are affected by CLL/SLL.
COPIKTRA’s mechanism of action
COPIKTRA is an inhibitor of PI3K-delta and PI3K-gamma enzyme isoforms, which are known to support the growth and survival of malignant B-cells.
The drug blocks a number of cell-signalling pathways, including B-cell receptor signalling and CXCR12-mediated chemotaxis of the cancer cells. In addition, it inhibits CXCL12-induced T-cell migration and M-CSF and IL-4 driven M2 polarisation of macrophages.
COPIKTRA is available in the form of 25mg and 15mg capsules.
Clinical trials on COPIKTRA
The FDA’s approval of COPIKTRA was based on data from a randomised, multi-centre, open-label trial (NCT02004522) that compared duvelisib to ofatumumab in patients with relapsed or refractory CLL or SLL.
The estimated median progression-free survival (PFS) was the primary endpoint, as assessed by an independent review committee (IRC).
The trial enrolled a total of 196 patients that received at least two prior therapies. Of these, 95 were randomised to duvelisib and 101 to ofatumumab. The patients were administered with 25mg of duvelisib initially over a 21-day treatment cycle followed by 28-day treatment cycles for up to 18 cycles.
The estimated median PFS was 16.4 months in the duvelisib arm and 9.1 months in the ofatumumab arm, with the overall response rate (ORR) of 78% and 39% for the two arms respectively, according to the IRC.
The drug’s approval for FL indication was based on a single-arm, multi-centre trial that enrolled 83 patients with FL. The patients were administered with 25mg of duvelisib for a median duration of five months. An ORR of 42% was achieved in the trial with 41% of patients experiencing partial responses as determined by an IRC.