Cosentyx (secukinumab), discovered and developed by Swiss pharmaceutical company Novartis International, is the first interleukin-17A (IL-17A) inhibitor drug approved for the treatment of moderate-to-severe psoriasis in patients aged six years and older who may benefit from systemic therapy or phototherapy.
It is also indicated to treat active psoriatic arthritis (PsA) in patients aged two years and older, active enthesitis-related arthritis (ERA) in patients aged four years and older, and adults with active ankylosing spondylitis (AS), active non-radiographic axial spondyloarthritis (nr-axSpA) and objective signs of inflammation. The drug is also indicated to treat hidradenitis suppurativa (HS).
Cosentyx is a clear to opalescent, colourless to slightly yellow solution available in 150mg/ml strength in a single-use Sensoready® pen and prefilled syringe for injection. It is also available as 150mg, lyophilised powder in a single-use vial for reconstitution for use by a healthcare professional only for subcutaneous administration.
Regulatory approvals for Cosentyx
The US FDA approved Cosentyx intravenous (IV) formulation for treating adults with PsA, AS and nr-axSpA in October 2023. Cosentyx is the only treatment approved as an IV formulation that selectively targets and blocks interleukin-17A antagonists. It is also the only non-tumour necrosis factor alpha (TNF-α) IV choice present in these indications.
In the same month, FDA approved Cosentyx as the first new biologic treatment option for hidradenitis suppurativa (HS) patients.
It was first approved by the European Commission (EC) in January 2015 as a first-line systemic treatment for moderate-to-severe plaque psoriasis in adult patients requiring systemic therapy.
The US FDA also granted approval for Cosentyx for the treatment of plaque psoriasis in the same month. The Dermatologic and Ophthalmic Drugs Advisory Committee unanimously recommended approval of secukinumab by the FDA in October 2014. The FDA approval was based on ten phase II and III clinical trials conducted on approximately 4,000 adult patients with moderate-to-severe plaque psoriasis.
Cosentyx is also approved in Australia for the treatment of moderate-to-severe plaque psoriasis and in Japan for the treatment of psoriasis and PsA.
In February 2018, the FDA approved a label update for Cosentyx. This marks the first approval for an IL-17A inhibitor in treating moderate-to-severe plaque psoriasis. The revised label encompasses Cosentyx data related to moderate-to-severe scalp psoriasis, a challenging form of the disease affecting about half of all psoriasis patients.
The EC approved Cosentyx (secukinumab), used only or in combination with methotrexate, in the juvenile idiopathic arthritis groups of ERA and juvenile PsA in patients six years and older who had not responded to or were intolerant to conventional therapy, in June 2022.
Psoriasis causes, symptoms, and effects
Psoriasis is an autoimmune disease characterised by thick and extensive skin lesions, called plaques, that cause intense itching, scaling and pain. People affected with psoriasis often lack physical and psychological quality of life.
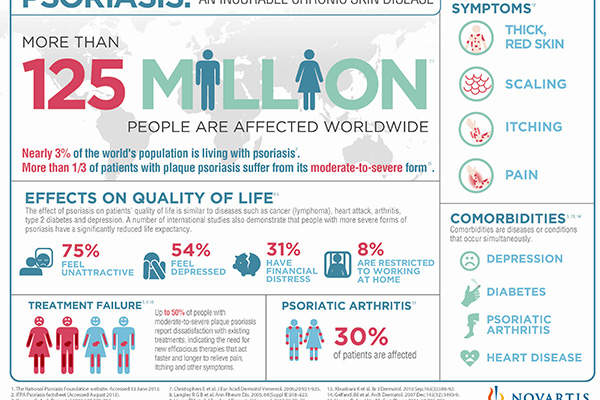
The devastating disease is estimated to affect up to 125 million people or 3% of the world’s population, with Europe and the US accounting for 3.7 million and 7.5 million psoriasis patients respectively.
Psoriasis severely affects everyday life and is associated with an increased risk of chronic illnesses.
HS is a systemic and chronic skin disease characterised as recurring boil-like abscesses that are often painful. The lumps may burst into open wounds and cause irretrievable markings, usually in the most intimate parts of the body. Disease diagnosis and progression may take a long time. The disease is estimated to affect one in 100 people worldwide.
Secukinumab’s mechanism of action
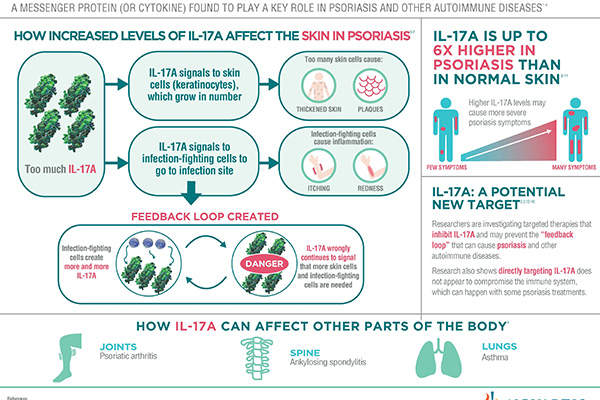
Secukinumab is a human monoclonal antibody, which works by inhibiting the action of IL-17A, a protein found in high concentrations in the skin of those affected by psoriasis. The protein is often the preferred target in investigational therapies.
Secukinumab is the only first-line treatment for psoriasis in Europe, whereas all available treatments for the ailment are recommended for second-line systemic therapy.
Doctors can use the drug as a potential alternative to numerous existing treatments that cause significant side effects.
Clinical trials on secukinumab
The EU approval is based on the results from a phase III clinical study known as Clear. The study enrolled patients with moderate-to-severe plaque psoriasis.
Secukinumab was also compared to Stelara (ustekinumab), an existing treatment for plaque psoriasis, and was found to be superior in clearing the skin of the affected patients.
Secukinumab demonstrated significant and consistent results including high skin clearance, sustained efficacy, safety and superiority to similar medicines such as Enbrel (etanercept) in all clinical trials.
In two clinical trials, Fixture and Eraser, approximately three-quarters of patients treated with secukinumab 300mg achieved clear skin on the Psoriasis Area Severity Index (PASI 100) or almost clear skin (PASI 90) during the first 16 weeks of treatment. The same results were observed in the majority of patients up to 52 weeks with continued treatment.
The FDA reviewed four placebo-controlled pivotal clinical studies: Fixture, Erasure, Feature and Juncture. The studies examined the safety and efficacy of secukinumab 300mg and 150mg in patients with moderate-to-severe plaque psoriasis. It also showed positive results from the second week.
Approximately 50% of the symptoms were reduced by the third week of using secukinumab, compared to seven weeks for patients administered with Enbrel (etanercept) in the Fixture study.
The studies met all primary and secondary endpoints including PASI 75, PASI 90 and Investigator’s Global Assessment modified 2011 (IGA mod 2011) 0/1 responses, and demonstrated significant results at week 12.
PASI measures the severity of the plaques including redness, scaling and thickness, with the efficacy of treatment assessed by the reduction of scores from the baseline. PASI 75 indicates 75% reduction and PASI 90 indicates 90% reduction, representing the highest skin clearance.
The majority of patients who achieved PASI 75 response and IGA mod 2011 0 / 1 at week 12 maintained the same response until week 52 with continued treatment.
The drug reported a favourable safety profile with similar adverse reactions such as nasopharyngitis, headache, diarrhoea, itching and upper respiratory infection in all treatment arms (300mg and 150mg).
Additional clinical studies on secukinumab
The FDA approval of Cosentyx for HS was based on the data from the two pivotal Phase III programmes: SUNSHINE and SUNRISE.
In the trials, a higher percentage of patients receiving Cosentyx 300mg either biweekly or every four weeks achieved a Hidradenitis Suppurativa Clinical Response (HiSCR50) compared to those on a placebo. HiSCR50 score indicates if a patient has achieved a 50% reduction or more in the total abscesses and inflammatory nodules associated with HS.
Cosentyx for HS is sanctioned at a 300mg dosage, administered every four weeks, with the flexibility to escalate to a biweekly schedule if the patient’s response is inadequate.
In comparison with placebo, the SUNSHINE and SUNRISE studies, spanning 16-week and 52-week treatment periods, revealed that the onset of Cosentyx action occurred as early as Week 2.
The efficacy progressively improved, reaching its peak at Week 16 and sustained through Week 52. The safety profile observed in the HS trials for Cosentyx aligned with its established safety profile from plaque psoriasis trials, re-inforcing the distinct safety characteristics of Cosentyx.
The most common adverse events reported during the clinical trials were cold, diarrhoea and upper respiratory tract infection.