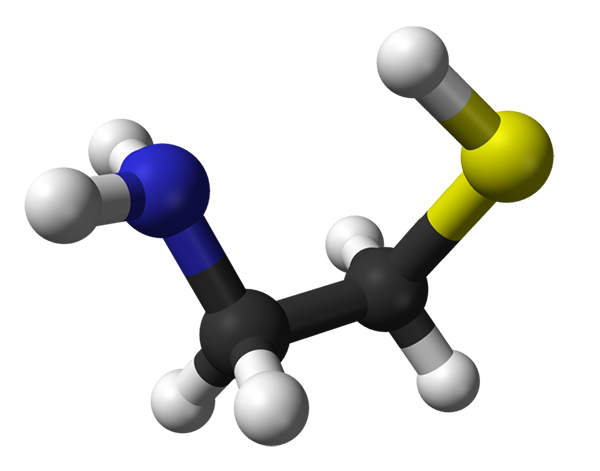
Cystaran (cysteamine hydrochloride) is a cystine-depleting agent indicated for the treatment of cystinosis. It has been developed by Sigma-Tau Pharmaceuticals in collaboration with National Institutes of Health (NIH).
In October 2012, Cystaran was approved by the US Food and Drug Administration (FDA) for the treatment of corneal cystine crystal accumulation related to cystinosis. The drug was also given orphan designation in the US by the FDA.
Cystinosis disease causes and effects
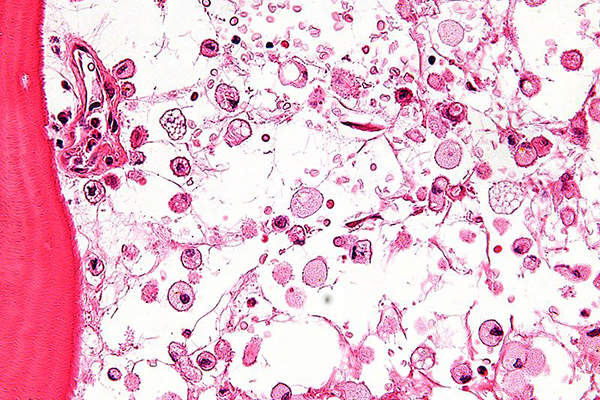
Cystinosis is a rare genetic disorder caused by lysosomal dysfunction. The disease causes the amino acid cystine to build up in various organs of the body. It slowly destroys liver, muscles, brain, eyes, and kidneys.
The build-up of cystine in the cornea can result in a change in visual acuity, foreign body sensations, squinting, photophobia and corneal haziness. It can also cause weakness of muscles, rickets, hypothyroidism and difficulty in swallowing.
The Cystinosis Research Foundation estimates that the disease affects about 500 people in the US and over 2,000 people across the world.
Cystaran mechanism of action
Cystaran contains cysteamine, a cystine-depleting agent that reduces the cystine content of cells in patients with cystinosis. The drug works by converting the amino acid cystine in to cysteine, which plays a significant structural role in proteins.
The drug is meant for topical use, and is administered as an ophthalmic solution at a recommended dose of 0.44% for every one hour.
Cysteamine hydrochloride clinical trials and results
Phase I clinical trials on Cystaran were conducted between May 1998 and March 2001. The study was conducted in Maryland, US, on 51 subjects. The purpose of the study was to evaluate the safety and efficacy of Cystaran in the treatment of corneal cystine crystal accumulation in cystinosis.
Phase II clinical trials on Cystaran were conducted between December 1999 and February 2001. The trial was a randomised, multicentre study which was conducted on 30 subjects. Patients received Cystaran as drops in one eye, and another current formulation of drops in the other eye for six months of safety study, and one year of efficacy study.
The FDA approval for Cystaran was based three Phase III controlled clinical trials conducted by Sigma-Tau and NIH. The studies enrolled about 300 cystinosis patients with corneal cystine crystal accumulation. The primary endpoint of the study was finding the response rate of eyes that had the photo-rated Corneal Cystine Crystal Score (CCCS) decreased by at least one unit.
Cystaran’s effectiveness in treating corneal cystine crystals was proven in these controlled clinical studies.
The first study’s results included data from three smaller studies. The response rate for eyes with a lower baseline of CCCS <1 was at 13% while it was at 32% for eyes with CCCS >1.
The second study evaluated the patients with a baseline of CCCS >1. The response rate was 67%. The third and last study evaluated patients with a baseline of CCCS >1. The response rate in this study was 33%.
The adverse effects found during the clinical studies included redness, sensitivity to light, headache, visual field defects, eye irritation, and pain.
Marketing Cystaran in the US
Sigma-Tau received FDA approval for marketing Cystaran exclusively in the US for seven years. It is the first drug approved for the treatment of cystinosis. It was developed through financial help from FDA which was granted under the Orphan Drug grant.
The Sigma-Tau Group is headquartered at Gaithersburg in Maryland, US. The company develops and manufactures medicines for rare diseases. It focuses on developing drugs for genetic disorders, cancers, and kidney diseases.