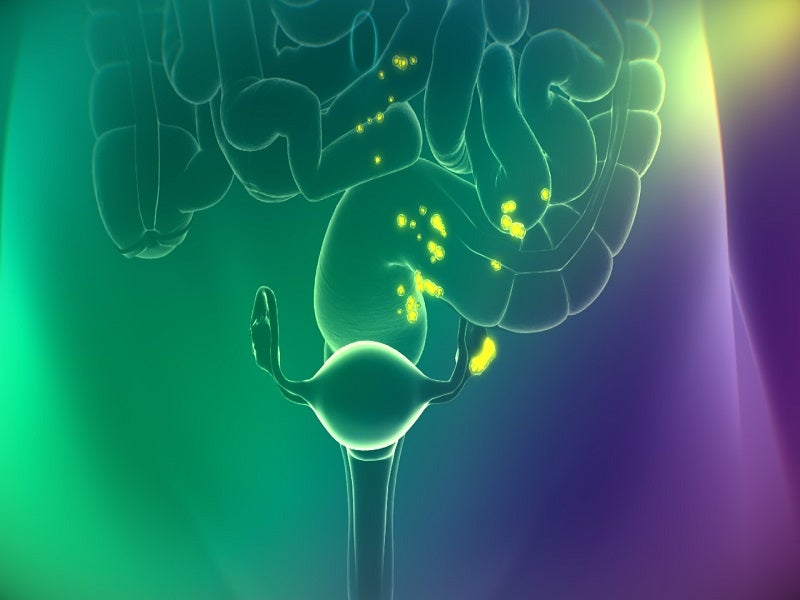
Cytalux™ (pafolacianine) is an optical imaging drug intended to serve surgeons as an adjunctive tool to identify and illuminate ovarian cancer lesions, even those located in usually difficult to detect areas, during surgery in adult patients.
Developed by US-based biotechnology company On Target Laboratories, Cytalux is available in a single-dose vial as a sterile, non-pyrogenic, dark bluish-green, clear aqueous solution of 3.2mg/1.6ml (2mg/ml) of pafolacianine for intravenous administration. The drug allows surgeons to quickly locate and accurately remove tumours.
In March 2021, On Target Laboratories received $21m in an extended Series B fundraising round to support the development and commercialisation of pafolacianine sodium injection. The funding round was backed by Johnson & Johnson Innovation and included JJDC, HIG Capital, Elevate Ventures, The Hurvis Group and 3B Future Health Fund.
Regulatory approvals for Cytalux
In December 2020, On Target Laboratories submitted a new drug application (NDA) for Cytalux as an adjunct for identifying ovarian cancer during surgery to the US Food and Drug Administration (FDA). The NDA was accepted for priority review in March 2021.
The drug received approval for the condition in November 2021. It had previously received both orphan drug and fast track designations from the FDA for the identification of ovarian cancer during surgery.
Cytalux is also being investigated for lung cancer in a Phase III clinical study, ELUCIDATE, under fast-track designation.
Ovarian cancer causes and symptoms
Ovarian cancer originates in the cells in the ovaries. The folate receptor found in cell membranes is overexpressed in most epithelial ovarian cancers. The cells reproduce rapidly and invade and destroy healthy bodily tissue.
Ovarian cancer is the second most common gynaecologic cancer in the US, killing more females than any other type of female reproductive system cancer.
Only 20% of ovarian cancers are detected early as they sometimes show no symptoms or mimic other common bladder, bowel or gastrointestinal disorders.
Signs and symptoms of ovarian cancer may include abdominal swelling, quickly feeling full after eating, weight loss, discomfort in the pelvic region, fatigue, back pain, constipation and frequent urination.
Cytalux’s mechanism of action
Cytalux is a fluorescent drug that binds to the folate receptor (FR) overexpressed in ovarian cancer. It illuminates during surgery under the near-infrared light after conventional intravenous (IV) administration as early as one hour before surgery.
The drug binds with a 1nM affinity to FR-expressing cancer cells, internalises through receptor-mediated endocytosis and accumulates in FR-positive cancer tissues.
Clinical trials on Cytalux
Cytalux’s safety and efficacy were demonstrated in a randomised, multi-centre, open-label trial, which enrolled 178 women diagnosed with ovarian cancer or with a high clinical suspicion of ovarian cancer, who were scheduled to have surgery.
Among the 178 women, 134 were treated with both normal light imaging evaluation and fluorescence imaging evaluation during surgery.
The primary outcome measure was the percentage of patients with at least one assessable ovarian cancer lesion confirmed by central pathology detected with Cytalux, under fluorescent but not under normal light or palpation, and which was not otherwise identified for excision before surgery. The secondary endpoint was the patient false positive rate.
In the study, 26.9% (N=36) of women demonstrated at least one malignant lesion that was undetected by normal visual or tactile assessment.
The false-positive rate at the patient level of Cytalux with near infra-red (NIR) fluorescent light in detecting ovarian cancer lesions was 20.2%, verified by central pathology.
The most common adverse effects of Cytalux reported in patients during the clinical trial were infusion-related events such as nausea, vomiting, stomach pain, flushing, dyspepsia, chest discomfort, itching and hypersensitivity.