Darapladib is a selective LpPLA2 inhibitor under development by GlaxoSmithKline (GSK). The drug is targeted at coronary heart disease (CHD) and is in Phase III development. Darapladib symbolises a new class of therapeutic agents that target atherosclerosis, the leading cause of CHD.
Coronary heart disease
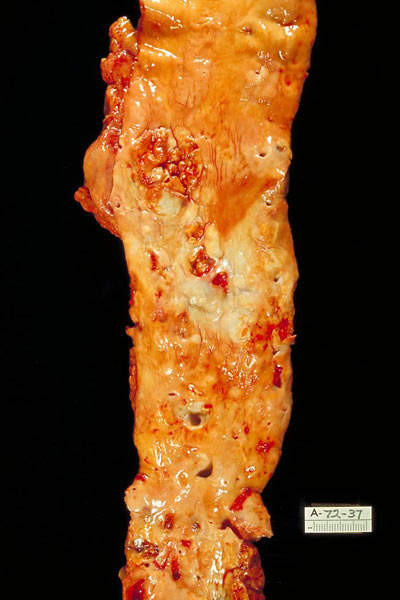
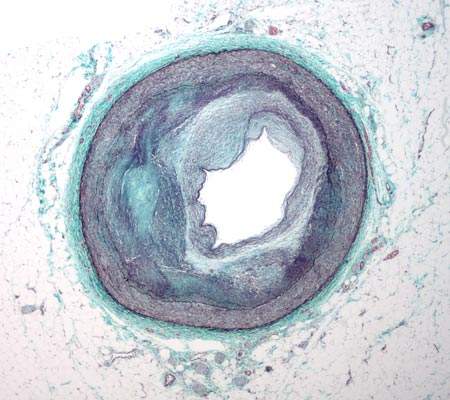
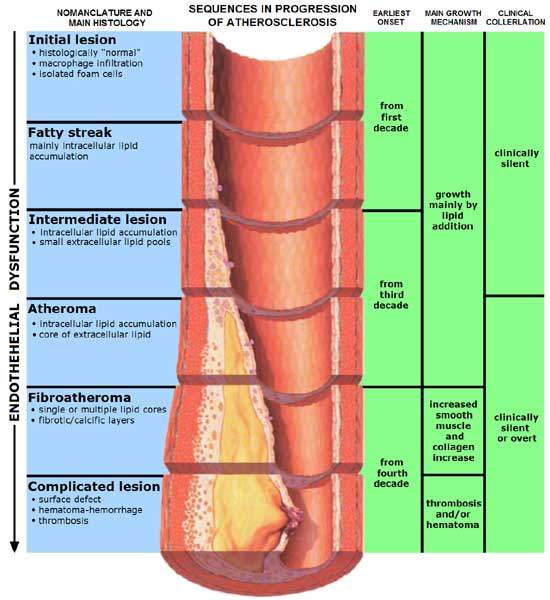
CHD is caused by a condition known as atherosclerosis. The condition occurs when a fatty material, calcium and scar tissue or plaque (soft interior consisting of cholesterol) forms in the coronary arteries.
As a result the arteries become narrow, decreasing the flow of oxygen-carrying blood to the heart. When blood flow decreases or stops it leads to chest pain, shortness of breath, heart attack and other conditions.
CHD is caused by various factors. People who have had incidence of CHD in their family are more at risk. Other factors include high cholesterol levels, high blood pressure, smoking, diabetes, lack of exercise, high-fat diet and emotional stress. CHD is also known as coronary artery disease.
CHD is the leading cause of death in men and women in the US. It is estimated that about 7.2 million people die every year from the disease worldwide. With more people leading a sedentary lifestyle and following a high-fat diet, the incidence of CHD is at an all-time high.
Darapladib as a treatment for CHD
Darapladib is an anti-atherosclerosis agent that inhibits the lipoprotein-associated phospholipase A2 (Lp-PLA2) enzyme. Despite using a wide range of cholesterol-lowering drugs, many people continue to be at risk of developing CHD and suffer heart attacks and stroke.
Research has indicated that presence of high levels of Lp-PLA2 in the coronary plaques formed due to atherosclerosis is responsible for the increase in the incidence of CHD. The Lp-PLA2 enzyme is present in large quantities in the blood and necrotic core of plaques. The enzyme plays a major role in the inflammation of plaques, putting them at risk of rupture, which leads to heart attacks and strokes.
Darapladib works to inhibit the activity of the Lp-PLA2 enzyme. It helps in preventing the expansion of the necrotic core of plaques, safeguarding them from rupture. This, in turn, helps lower the risk of CHD and other coronary diseases.
Clinical trials demonstrate darapladib’s potential
In December 2009, GSK announced that it has initiated a second Phase III clinical study to test the safety and efficacy of darapladib in treating acute coronary syndrome (ACS). Lack of sufficient blood to the heart of ischemia is the cause of ACS.
The Phase III randomised, placebo-controlled, double-blind study, The Stabilisation of pLaque Using darapladib -Thrombolysis In Myocardial Infarction 52 (SOLID-TIMI 52), will include 11,500 patients from 40 countries.
The first Phase III study, STabilisation of Atherosclerotic plaque By Initiation of darapLadIb TherapY (STABILITY), is testing the safety and efficacy of darapladib in people suffering from chronic coronary artery disease.
The STABILITY study is being conducted in men and women suffering with chronic CHD. The study was started in December 2009 with 15,000 people from 39 countries.
Phase III trials for darapladib were initiated after positive results from a year-long Phase II clinical trial in September 2008. The Phase II study, Integrated Biomarkers and Imaging Study-2 (IBIS-2), was a one-year worldwide, randomised, placebo-controlled, parallel-group trial conducted in 330 individuals suffering from angiographically recognised CHD. The study showed that use of darapladib stopped extension of the necrotic core of plaques.
Marketing commentary
New approaches and several advances in cardiovascular medicine have not been able to address the increased deaths and risks posed by CHD. Darapladib presents a new approach towards treating CHD by addressing its main cause, atherosclerosis.
The drug is expected to be a substantial addition to GSK’s pipeline. If approved, darapladib will significantly boost GSK’s revenues and also have very few competitors in the market.